Facile and Selective Synthesis of ZnO Hollow or Crumpled Spheres and Their Photocatalytic Degradation Activities
Article information
Abstract
Hollow or bumpy ZnO structures with micrometer-size features have been investigated as photocatalysts for water purification due to their high surface area available for reaction with harmful organic molecules and relatively large size for easy separation after finishing the photocatalytic reaction. In this study, selective synthesis of ZnO hollow or crumpled microspheres was performed using a simple and versatile ultrasonic spray pyrolysis process with various zinc precursors. The morphologies, phases, specific surface areas, and optical properties of the microspheres were characterized using X-ray diffraction, scanning electronic microscopy, nitrogen adsorption-desorption isotherms, and UV-vis spectroscopy. In addition, the mechanism underlying the formation of different morphologies and their photocatalytic activities were systematically investigated.
1. Introduction
Zinc oxide (ZnO) is a versatile material due to its unique material properties and is used in diverse applications because it provides electronic, photonic, and spin-based functionality through its wide band gap (3.37 eV) and large exciton binding energy of ~ 60 meV.1,2) These properties are particularly useful in applications such as piezoelectric transducers, optical coatings, gas sensors, photocatalysts, and photovoltaics. In addition, photocatalytic degradation of organic pollutants is an efficient remediation method compared with traditional advanced oxidation processes (AOPs) such as ozone, hydrogen peroxide, and UV light treatments. 3,4) To meet the wider application and higher activity demands for environmental remediation, developing advanced photocatalysts with high specific surface areas has become increasingly important.
ZnO nanostructures with high specific surface area including nanoparticles,5) nanowires,6) nanofibers,7) nanosheets, 8) and hierarchical forms9) have been developed to achieve improved photocatalytic activity. However, nanoscale photocatalysts tend to agglomerate, minimizing its surface energy and leading to decreased photocatalytic efficiency. Moreover, since dispersed nanoscale photocatalysts in waste media are hard to collect after decomposition of organic pollutants, it remains in the mixture as a pollutant itself. Hollow or bumpy ZnO structures with sub-micrometer-size features are particularly attractive as photocatalysts for a water purification due to their high surface area available to react with harmful organic molecules and large size for easy separation after finishing the photocatalytic reaction compared to microspheres and nanoparticles.
Ultrasonic spray pyrolysis (USP) is an aerosol processing method that can be used to produce relatively monodispersed sub-micron particles in a simple and continuous process using inexpensive precursors.10–12) This allows for the industrially-scalable production of various metal and oxide powders. Conventional USP processes generally synthesize a sub-micron or micrometer sized particle because the reduction of initial droplet size using ultrasound is limited. However, the size and morphology of the powder synthesized by USP can be readily modified by changing the type of precursor salt, which has distinct impacts on the decomposition and crystallization rate during pyrolysis.
In this study, controllable synthesis of sub-micron hollow and bumpy ZnO powders was achieved by changing the precursor for USP. The influence of precursor solution types (aqueous zinc nitrate and zinc acetate) on the morphological, structural, and optical characteristics of the ZnO samples was also systematically investigated. In addition, the mechanism by which the morphological differences occur are discussed. Finally, the photocatalytic activity of the synthesized ZnO powders were evaluated by monitoring the degradation of rhodamine-B under irradiation with a xenon lamp.
2. Experimental Procedure
All the reagents and solvents were purchased from Sigma-Aldrich and used directly without further purification. The starting solutions for USP were prepared by dissolving zinc nitrate hexahydrate (Zn(NO3)2·6H2O, 99.9%) and zinc acetate dihydrate (Zn(CH3COO)2·2H2O, 99.9%) to concentrations of 100 mM in distilled water. The prepared precursor solutions were ultrasonically nebulized at a frequency of 1.7 MHz into microdroplets, which were then carried by a gas flow into a tube furnace where solvent evaporation and precursor decomposition occurred producing ZnO powders. The furnace with a quartz tube (30 mm inner diameter and 500 mm long) was preheated to 500, 700, and 900°C, and compressed pure oxygen was used as a carrier gas at a flow rate of 2 L/min. The ZnO powders were collected by the incorporation of filter paper (Qualitative Filter Paper, 5 – 8 mm, Hyundai Micro Co., Ltd., Korea) into the carrier gas stream. For the sake of simplicity, the samples prepared from Zn(NO3)2 and Zn(CH3COO)2 at various temperatures are denoted as ZnO-N(temp.) and ZnO-A( temp.), respectively.
The crystal structures of the powders were characterized by X-ray diffractometry (XRD, X’Pert3 Powder, PANalytical, Netherlands) with a radiation wavelength of λ = 1.54056 Å. XRD patterns were obtained from 2θ = 20 to 80° at a scan rate of 4° min−1. Microstructural characterization of the ZnO powders was performed by field emission scanning electron microscopy (FE-SEM, S-4800, Hitachi, Japan). The decomposition and crystallization behavior of the precursors was monitored using thermogravimetric analysis and differential scanning calorimetry (TG/DSC, STA409PC, Netzsch). The specific surface area of the powders was determined using the Brunauer-Emmett-Teller (BET) method from the N2 adsorption isotherm. Absorption spectra were measured from 200 – 900 nm with an ultraviolet/visible spectrophotometer (UV-vis DRS, UV-2600, SHIMADZU, Japan).
The photocatalytic activity of the ZnO powders was studied by measuring the photodegradation rate of rhodamine B (Rh B). Their activity was confirmed with the decolorization of Rh B through photocatalytic degradation based on the Beer-Lambert law13) which is the linear relationship between absorbance and concentration of an absorbing species. The photocatalytic experiments were performed by adding 0.10 g of the ZnO powder to 100 mL of a 0.5 × 10−5 M Rh B aqueous solution. The mixture was kept in the dark for 30 min to reach the adsorption-desorption equilibrium between the photocatalyst, Rh B, and water before irradiation. The photocatalytic experiment was performed using a solar simulator equipped with a 300 W xenon lamp and an Air Mass 1.5 Global Filter simulating solar radiation reaching the surface of the earth at a zenith angle of 48.2°. The mixture was irradiated using the xenon lamp with magnetic stirring in a water bath at 25°C. A series of aliquots were withdrawn at selected times for analysis and were centrifuged before measurement. The residual concentration of Rh B in each sample was determined by UV-vis spectrometry (UV-vis DRS, UV-2600, SHIMADZU, Japan).
3. Results and Discussion
Figure 1 shows the XRD patterns of the powders synthesized by USP of a 100 mM aqueous solution of Zn(NO3)2 and Zn(CH3COO)2 at various temperatures. The diffraction peaks can be indexed according to the wurtzite ZnO structure (JCPDS card No. 36-1451) except for some peaks in the ZnO-N(500) spectrum which match zinc hydroxynitrates (Zn3(OH)4(NO3)2, JCPDS card No. 52-0627) as shown in Fig. 1(A). The diffraction peaks at 31.7, 34.4, and 36.3° correspond to the (100), (002), and (101) planes, respectively. The intensities of the diffraction peaks increased with pyrolysis temperature suggesting that the samples synthesized at higher temperatures are more crystalline. Except for ZnO-N(500), no impurity signals were detected, indicating the high purity of the products.

XRD patterns of the powders synthesized by the ultrasonic pyrolysis process with different zinc precursors; (a) zinc nitrate and (b) zinc acetate, at 500°C, 700°C and 900°C.
The morphologies of the ZnO-N and ZnO-A samples were examined by SEM, as shown in Figs. 2 and 3. All samples consisted of large amounts of polydispersed microspheres ranging from several hundred nanometers to 2 μm in diameter. The micrographs in Fig. 2 show the porous spherical shape of the ZnO-N powder, which consisted of multiple nanosized primary particles with a three-dimensional network. In addition, abundant mesopores were present between adjacent particles. As the synthesis temperature increased, particle growth due to sintering between the primary particles was observed and resulted in a smoother surface and shrinkage. On the other hand, the ZnO-A powders exhibited dimples and wrinkles, as shown in Fig. 3. The difference in morphology can be attributed to the different thermal and crystallization behaviors of the zinc precursors.
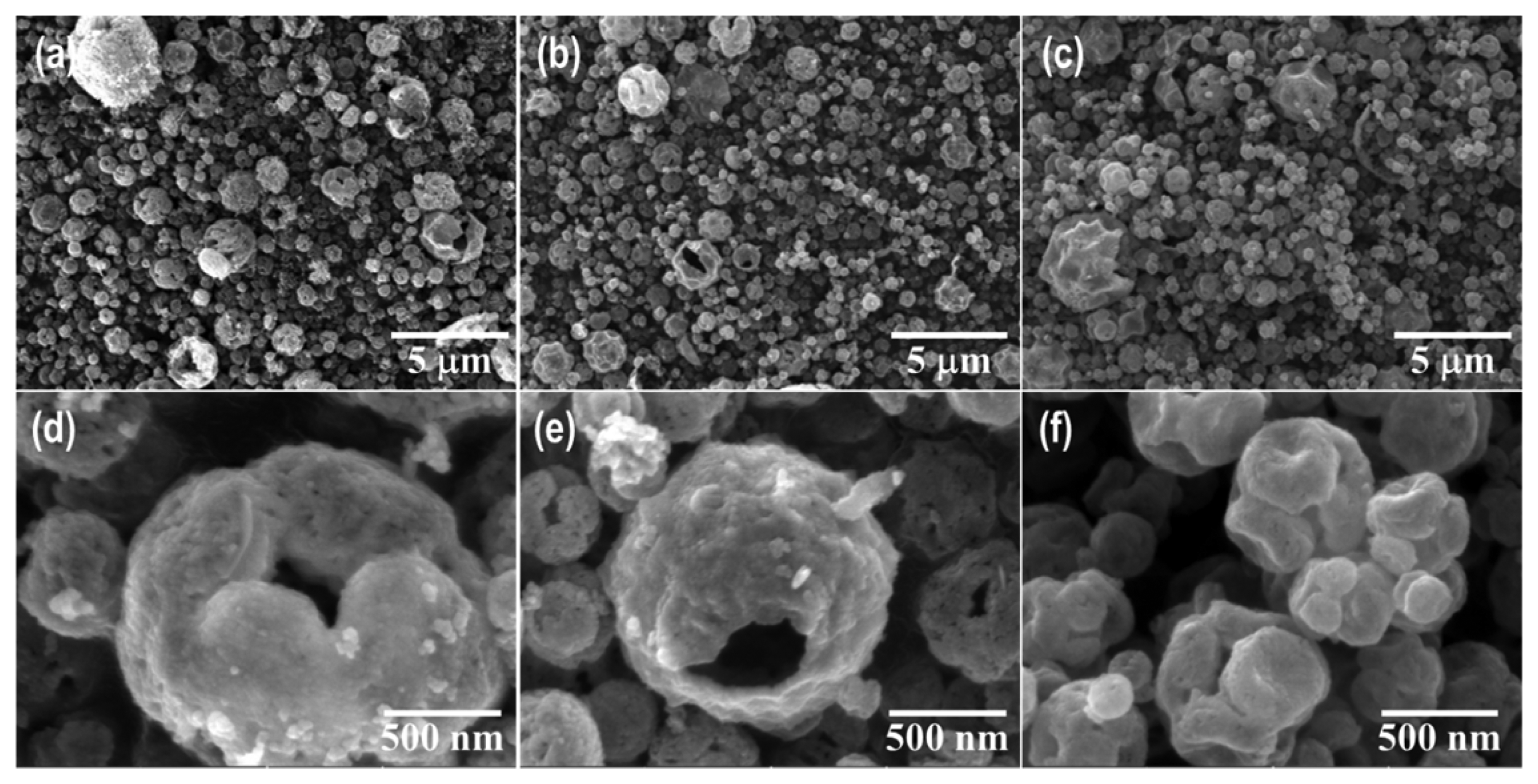
FE-SEM images of the powders synthesized by USP using a zinc nitrate salt at (a, d) 500°C, (b, e) 700°C, and (c, f) 900°C.
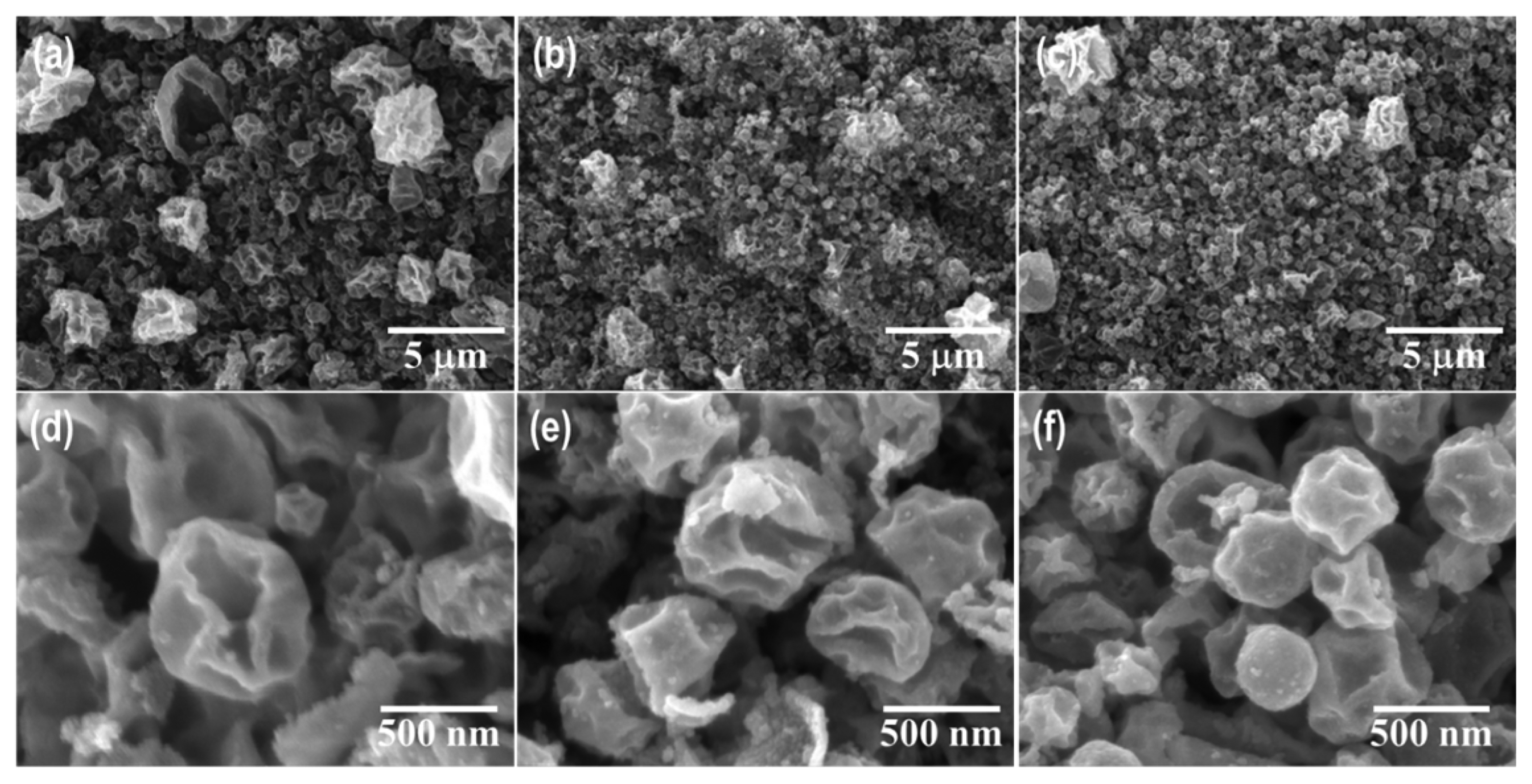
FE-SEM images of the powders synthesized by USP using a zinc acetate salt at (a, d) 500°C, (b, e) 700°C, and (c, f) 900°C.
The mechanism underlying the difference in morphology was identified by TG analysis of the zinc precursors. Fig. 4(a) shows the TG analysis results obtained during the thermal decomposition of zinc nitrate and acetate used for preparing the ZnO powders by USP. During initial stage below 200°C, the mass loss corresponds to water evaporation from both precursors without the loss of nitrate or acetate groups. Above 200°C, the total weight loss of the precursors differed according to the chemical formula but the overall weight loss trends were similar. However, as shown in Fig. 4(b), the DSC curves of the ZnO powders showed different behaviors, especially the final peaks of the zinc nitrate and acetate indicate an exothermic and endothermic reaction, respectively. This can be explained by the difference in thermal decomposition and crystallization behavior of each precursor. For zinc nitrate, the crystallization of ZnO occurs on the surface of dried precursor droplets as it slowly thermally decomposes. As a result, a core (precursor)/shell (ZnO) structure is formed during pyrolysis, which leads to the formation of hollow structures with an empty interior. This interpretation is consistent with the XRD analysis shown in Fig. 1(a). On the other hand, for zinc acetate the final reaction peak indicated an endothermic reaction, suggesting that crystallization occurs after quick thermal decomposition of the precursor. As a result, dried precursor droplet contraction caused by the rapid thermal decomposition of the precursor occurs, and ZnO subsequently crystallizes, creating a wrinkled morphology.
Figure 5 shows the nitrogen adsorption/desorption isotherms and pore-size distribution curves of the ZnO-N( 500), ZnO-N(700), and ZnO-A(500) samples. All three samples can be classified as type IV in the IUPAC classification with a distinct hysteresis loop in the range of 0.5 – 1.0 P/P0, which is characteristic of porous materials. The Brunauer-Emmett-Teller (BET) specific surface area of the ZnO-N(500), ZnO-N(700), and ZnO-A(500) samples are 13.18, 12.14, and 21.19 m2 g−1, respectively. Fig. 5(b) shows the Barrett-Joyner-Halenda (BJH) pore-size distribution plot for the N2 sorption isothermals of the prepared samples. It is clear that the ZnO-A(500) powder has a large number of pores in the macropore range between 10 and 100 nm which originated from its bumpy structure. For the ZnO-N(500) sample, the plot indicates that the powder has a relatively small number of pores that are large compared to those of the ZnO-A(500) powder. This resulted in the hollow structure of the ZnO-N(500) powder. The main pore size of the ZnO-N(700) sample was slightly smaller compared to the ZnO-N(500) powder and mesopores of approximately 5 nm was observed because of shrinkage by sintering of the primary particles. These analyses indicate that the ZnO-A(500) powder exhibited the largest surface area of all samples, and it is predicted to provide the best photocatalytic activity of all prepared samples.
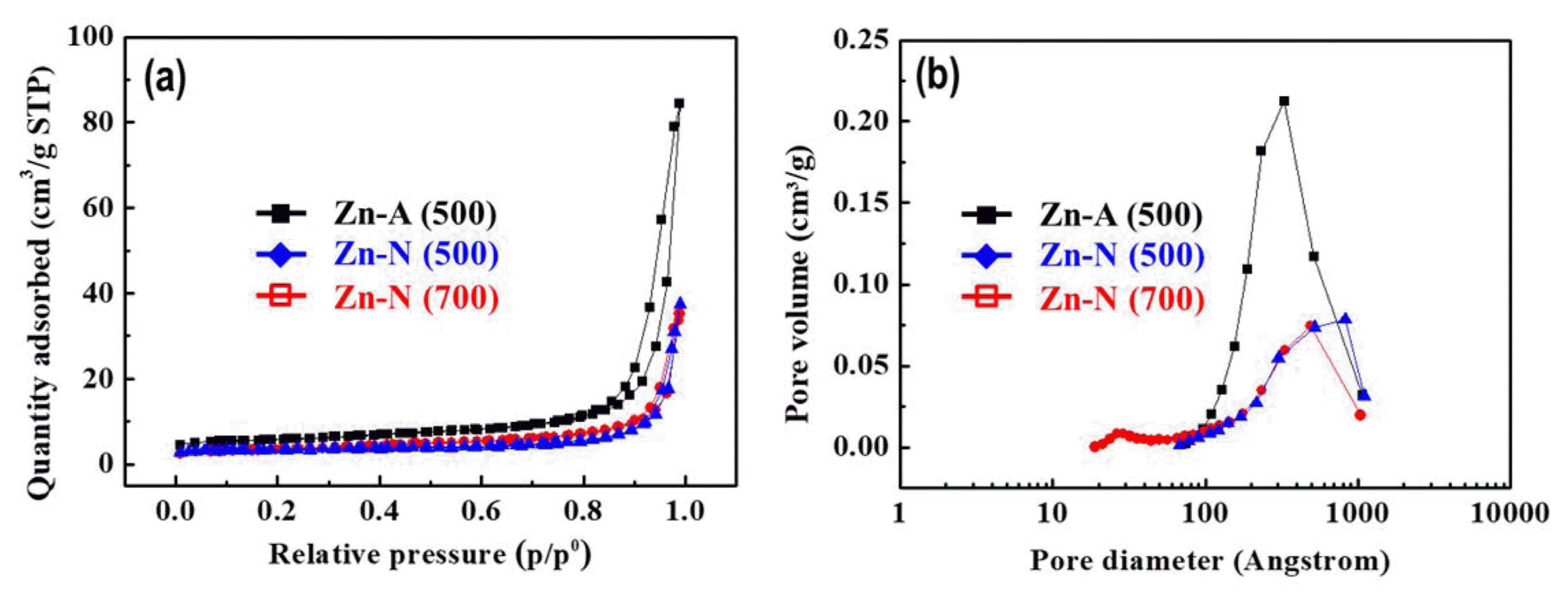
(a) N2 adsorption-desorption isotherms and (b) pore size distribution curves of the ZnO powders synthesized using different Zn precursors at 500 or 700°C.
Figure 6(a) shows UV-vis diffuse reflectance spectra for the synthesized powders. The absorption spectra exhibited an absorption shoulder at approximately 385 nm, which is similar to the absorption band of bulk ZnO. The band gap of the sample can be estimated from the spectrum in Fig. 6(A) using Kubelka-Munk theory.14) The converted Kubelka-Munk function graph is shown in Fig. 6(b) and extrapolating the linear sections of the curves gives the band gap of approximately 3.2 eV. This is consistent with the previously reported optical bandgap of 3.1 – 3.3 eV. The small band gap differences between each sample are thought to originate from the transitions involving extrinsic states, such as surface defects and oxygen vacancies.15)
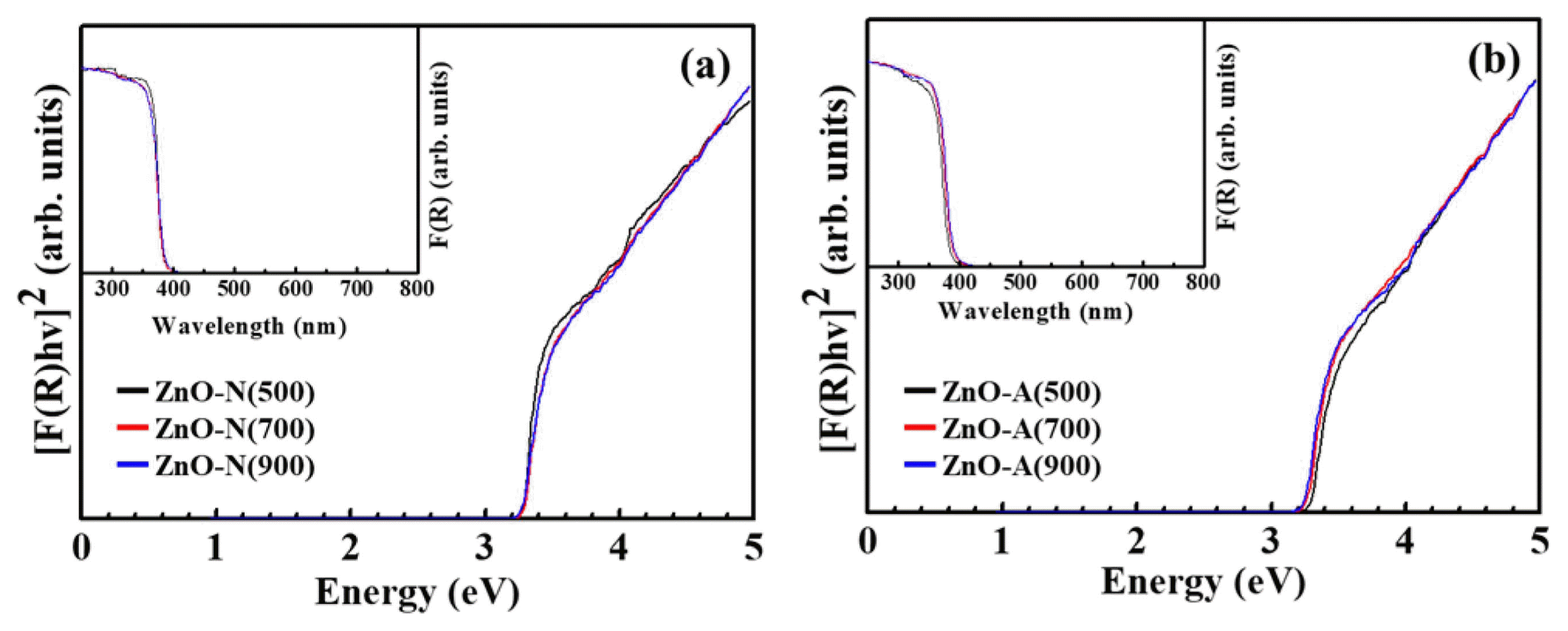
(Inset) UV-vis diffuse absorption spectra and plots of the transformed Kubelka-Munk function as a function of absorbed light energy of the ZnO powders synthesized using different Zn precursors; (a) zinc nitrate and (b) zinc acetate at 500, 700, and 900°C.
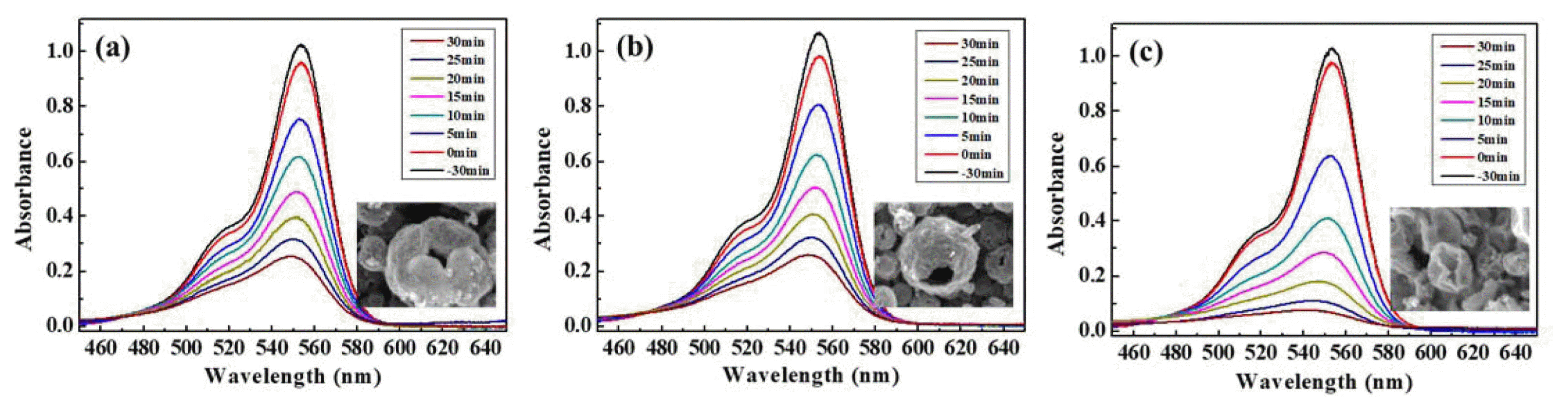
The samples synthesized from different precursors and temperatures exhibited different morphologies, and show correspondingly different photocatalytic performance. Fig. 7 shows the changes in the UV-vis spectra during the removal of aqueous Rh B by the ZnO powders. As shown in Fig. 7, the dark absorptions of the different samples are similar. The degradation percentage of the Rh B dye was approximately 75%, 73%, and 92% for the ZnO-N(500), ZnO-N(700), and ZnO-A(500) samples, respectively, after photo-irradiation for 30 min. This photocatalytic performance difference can be explained as follows. Photocatalytic activity is generally related to the morphology and surface area of the particles. High BET surface areas are favorable for increased photocatalytic activity. In this study, the specific surface area of the samples increased in the order of ZnO-A(500) < ZnO-N(500) < ZnO-N(700), as shown in Fig. 5. Thus, the ZnO-A(500) samples was expected to should show the highest photocatalytic activity.
4. Conclusions
In summary, we have demonstrated that USP using different zinc precursors provides an effective route for the selective synthesis of ZnO powder with hollow or bumpy shapes. The different morphologies have a significant influence on the specific surface area and photocatalytic activities of the prepared ZnO powders. TG-DTA analysis indicated that the morphology difference originated from the decomposition and crystallization behavior of the zinc precursors during pyrolysis. The ZnO powder with a bumpy structure synthesized from zinc acetate exhibited a higher specific surface area and pore volume than the powders with a hollow interior prepared from zinc nitrate, resulting in superior photocatalytic activity. This study demonstrates that various powder morphologies can be selectively synthesized with USP.
Acknowledgments
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT, and Future Planning (NRF-2015R1C1A1A02037745).