Color Variation in Color-shade Polycrystalline Zirconia Ceramics by the Atmosphere Controlled Firing
Article information
Abstract
Color shade variation was investigated in zirconia dental blocks, prepared using commercial powders. As a reference color-shade block we used the color indexes of A2, A3.5, A4 and B3, according to the VITA classical color scale. The zirconia powders for color shade blocks showed colors of white, yellow, pink and grey, respectively, after firing at 1530°C. The zirconia powders were mixed according to the recipe of color shade blocks and shaped at lower pressure using a uniaxial hydrostatic press. The shaped sample was inserted into a vinyl pack and sealed in a vacuum form machine. The shaped block samples were reshaped at 450 bar using an isostatic cold press and fired at 1530°C for three hours. In order to investigate the atmospheric color variation with firing temperature, the A2, A3.5, A4 and B3 sintered blocks were fired between 700°C and 1300°C under controlled atmosphere of pN2 and pO2. The surface color picture was taken using a smart phone camera and compared with the results obtained using the VITA classical color scale. Quantitative color index value, CIELAB, was measured using a color-meter. Above 800°C, the color darkness greatly increased with the increase of the reduction temperature and keeping time.
1. Introduction
Yttrium-stabilized zirconia ceramics as dental ceramics1–3) have been well developed and commercially utilized as typical dental materials for artificial teeth. In the earlier stages there were some difficulties in the mechanical stability such as stiffness and thermal stability due to the complex chemical situation in oral biology. In 2000, the dental application of zirconia ceramics was abruptly introduced and now zirconia ceramics are the best dental materials for use in veneers, implants, multi-connected bridges, and so on.4–6) The final issue in dental clinics is always how to match the color-shade of the artificial veneer surface with patient tooth color. For color shade-matching, the dentist asks the dental laboratory to do the work professionally. Conventionally dental lab artists have tried to reveal three-dimensional color situations of patient teeth using zirconia veneer. In recent technology7–10) developments, the materials process has been based on color shaded zirconia blocks and the veneer ceramics are obtained through CAD/CAM machining. Finally, dental lab artists coat the color film on the surface of the veneer ceramics using coloring glass; the coated veneer ceramics are then fired at intermediate temperature between 700°C and 800°C. For this purpose, zirconia ceramics such as A2, A3.5, or B3 are specially prepared and commercially supplied. In this research we show multicolor shade technology using unique color shaded zirconia ceramics through atmosphere-controlled firing; our results may contribute to simplifying the complicated coloring process in artificial teeth for dental patients.
2. Experimental Procedure
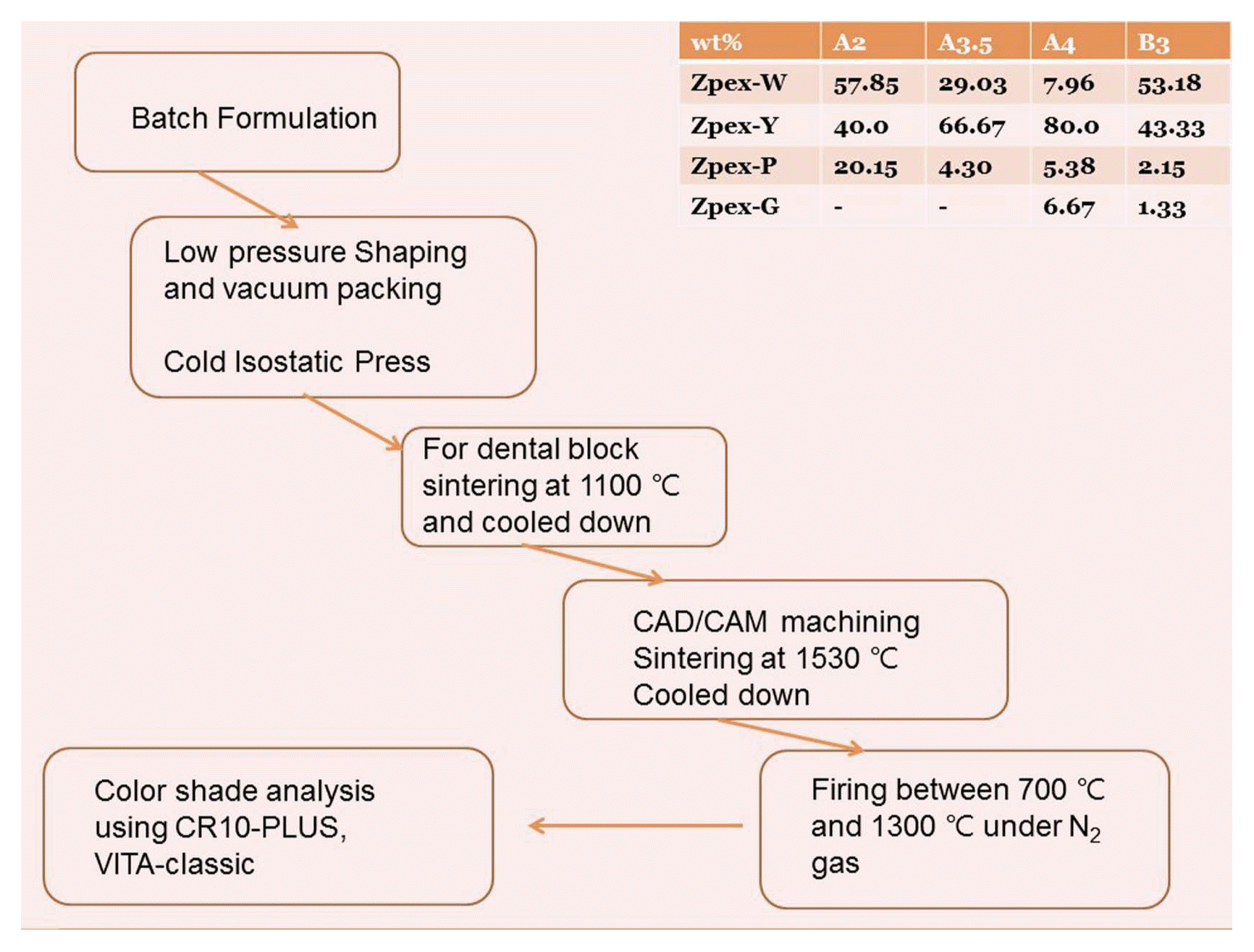
2.1. Preparation of color shade zirconia dental block
In this experimental process, we prepared color-shaded zirconia dental blocks A2, A3.5, A4 and B3, which were based on the VITA Classics color scale [VITA Zhanfabrik H. Rauter Gmbh & Co, KG D-79713 Germany], commercially used in dental laboratories. The dental blocks were prepared using commercial zirconia powders such as Zpex-White [Zpex-W], Zpex-Yellow [Zpex-Y], Zpex-Pink [Zpex-P], and Zpex-Gray [Zpex-G], which were supplied from TOSOH Co. in Japan.8,9) We prepared four kinds of color shaded zirconia dental blocks using the reported powder formulation recipes,8,9) shown in Fig. 1. The formulated powders were hand-mixed in a vinyl packing wrap and the packing wrap was tumbled using a pulverizer [FRITSCH Spartan, Germany]. Immediately the mixed powders were shaped using a stainless-steel mold with 12 cm diameter; molded powders were then pressed for 5 minutes in a hydraulic press under 0.013 ton/cm2. The shaped round sample was inserted into a vinyl wrap and the wrapped sample was sealed using a vacuum packing machine. The sealed sample was hydrostatically shaped at ~ 450 bar using an ICP [Isostatic Cold Press] machine [ISA-CIP-S30-200, ILSHIN Autoclave, Korea]. The shaped block was taken out of the sealed package and put into the furnace for sintering. In order to make multi-blocks of A2 and A3.5, the formulated zirconia powders for the A2 block were put into the above stainless-steel mold and tumbled using a pulverizer; then, the formulated powders for the A3.5 block were put on the surface of the A2 powders in the same mold. The whole mass of powder in each mold was moved to the uniaxial press. Using the uniaxial press, the multi-block sample was pressed for 5 minutes in a hydraulic press under 0.013 ton/cm2. Shaped round samples sealed with vinyl wrap were hydrostatically shaped using CIP.
The first firing was done at 1100°C for 3 h and then samples were cooled. Normally calcined blocks are used for CAD/CAM machining in dental labs. The machined blocks were finally sintered at 1530°C for 3 h and cooled. The sintered samples showed the corresponding color indexes for A2, A3.5, A4, and B3, respectively.
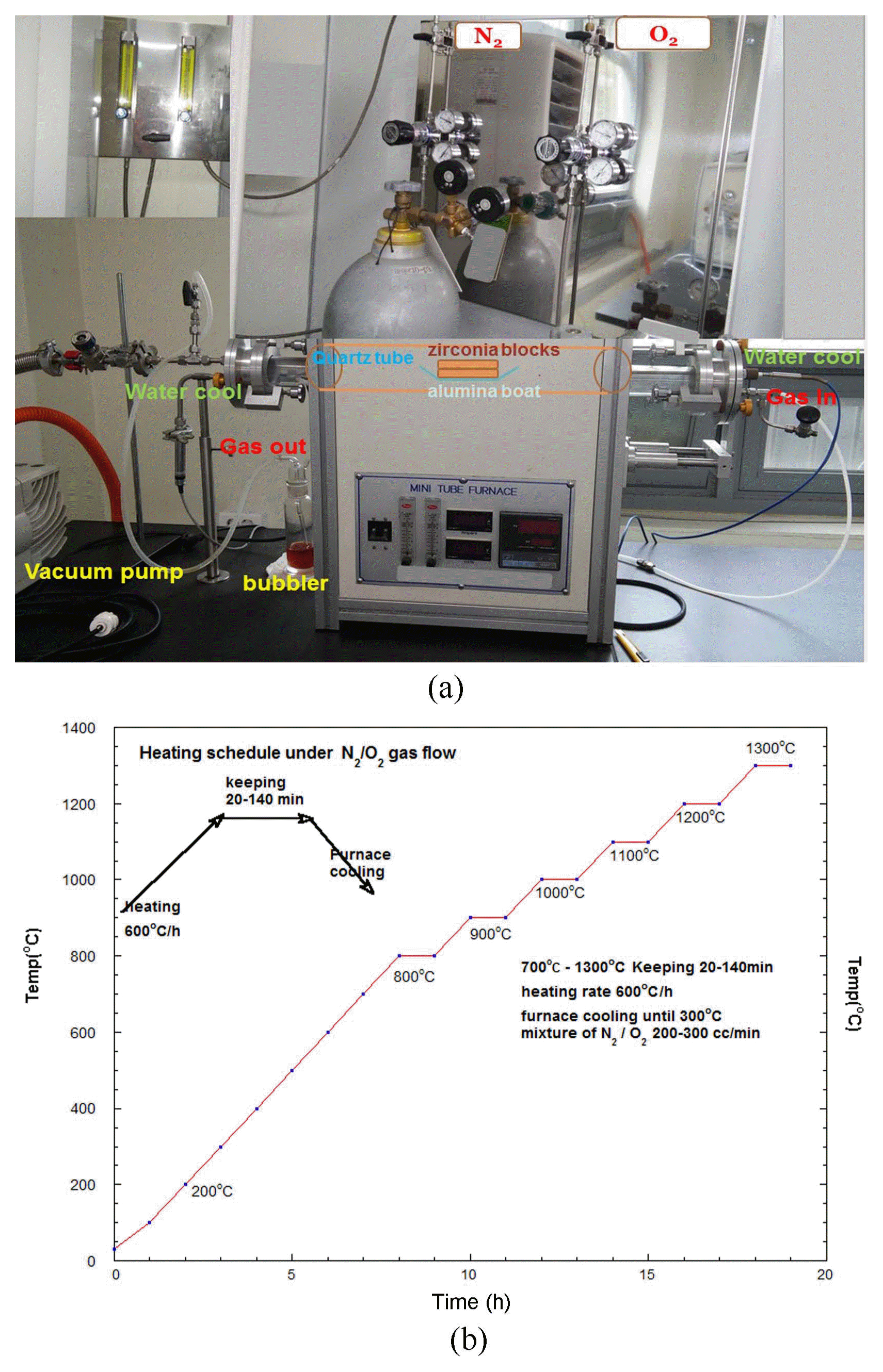
2.2. Color variation of color shade zirconia dental block using atmosphere-controlled firing
As shown in Fig. 2 the color-shaded zirconia blocks were put in the center region of the tube furnace and fired at intermediate temperature between 700°C and 1300°C, according to the firing schedule, under the proper atmosphere mixing of O2 and N2 gas. Critically, the fired samples showed volumetric color change according to the gas mixing ratio and the firing temperature schedule, such as heating, cooling, and holding times. Additionally, we tried to show a that three-dimensional color change can occur in the sample body if we can control the temperature schedule of the samples placed in the center of the longitudinal firing tube, as shown in Fig. 2. Occasionally we were able to show a two-dimensional color change along with distance displaced from the tube center, which means that there is a firing temperature gradient along with sample length. Additionally, we were able to observe gradual color change along the depth of the sample block. This finding can lead to novel coloring process technology for these color-shade dental zirconia blocks, which can be utilized in dental labs to fabricate color-shade blocks.
In zirconia ceramics, the white color base is too strong to reveal the color of added pigments.5) The pigment color disappears5–10) at the required firing temperature above 900 mixing of O C because the pigment element cannot be attached to the white YSZ powders, indicating the hard solid state interaction in 3YSZ powders with color pigment elements such as FeOx, CoOx and others. No stains or other colorants will adhere or bond to Zirconia ceramics. In dental laboratories, it is difficult to reveal the natural tooth color on a zirconia surface with its stark white color. Normally, artificial teeth are coated with glass enamel to a thickness between 0.001 mm and 1.0 mm. The color glass is coated on the surface of the porous zirconia block and samples are fired at 1380°C for 7 – 8 h. For good quality, this process is performed three times.
The amounts of FeOx and CoOx in our experimental blocks were under 1 wt% [Tosoh] and the color changing process was done through atmosphere firing. The purpose of this process is to investigate the color transition due to REDOX change11,12) of Fe and Co ions in the 3YSZ ceramic matrix. The color gradation was analyzed using the VITA Classic Scale and CR-10 PLUS. The solid-state interaction between the 3YSZ matrix and the coloring elements was analyzed using Raman spectroscopy.13–16)
2.3. Characterization
2.3.1. Microstructure and solid-state interaction
The microstructure of the atmospherically sintered zirconia block body was investigated and EDS was measured using Ultra High FE-SEM [Hitachi, SU-8220]. In order to analyze the color change due to the solid-state incorporation of Fe, Er, and Co components, Raman spectroscopy [NTEGRA, NT-MDT Russia] was performed for the fired samples.
2.3.2. Color shade matching
For color shade matching, color images were taken using a digital camera (Galaxy A800, Samsung Co.) before and after annealing. In order to estimate the color of the zirconia dental blocks, we used the VITA Classical color scale and measured the Lab color indices using a colorimeter [CR-10-plus, Minolta]. CIE Lab color indices17,18) were determined for the spectrum secured through this procedure by using the color analysis program embedded in the instrument. The L* value shows brightness, with red ~ green (more red color for a more negative number and greener color for a more positive number) in the case of a*, and blue ~ yellow (bluer color for a more negative number and more yellow color for a more positive number) in the case of b*. The color difference (ΔE) was calculated as ΔE = [(ΔL*)2(Δa*)2 (Δb*)2]1/2 using the Lab indices.
CIELAB Color Space used in dentistry
The Commission Internationale de l’Eclairage (CIE) developed the CIE 1976 L*, a*, b* color space, with the official abbreviation CIELAB.17,18) The L* coordinate or axis quantifies the lightness and has a scale from zero to 100. The a* axis represents redness-greenness, with zero being achromatic, a negative a* value representing more greenness than redness, and a positive a* value representing more redness than greenness. The b* axis represents yellowness-blueness, with zero being achromatic, a negative b* denoting more blueness than yellowness, and a positive b* denoting more yellowness than blueness. CIELAB was developed to create a more perceptually uniform color space that could be correlated with the visual appearance of colors. This color space is currently the most widely used color space in dental color research as the difference in colors can be calculated by measuring the Euclidean distance between two colors and because these color differences have been correlated with visual perception. Color difference is represented by the Greek symbol Δ (delta) and is described according to the following formula,
where x and y denote two different colors in the CIELAB color space and ΔE denotes the overall color difference between x and y. Due to the mathematical operations in the formula, total color difference is an absolute value and does not provide any information about the direction of the difference. Information about the direction of color difference is determined by the difference in each of the three CIELAB coordinates: ΔL*, Δa*, and Δb*, expressed respectively by the following formulas,
where x and y denote two different colors in the CIELAB color space. A disadvantage of CIELAB is that color differences in the a* and b* axes cannot be described in terms of a change of hue and chroma, making CIELAB less applicable in clinical settings.
VITA Classical guide
The VITA Classical (VITA Zahnfabrik, Bad Sackingen, Germany) shade guide is one of the most commonly used guides. It has a total of 16 shades that are divided into four groups based on hue. The manufacturer labels group A reddish brown, group B reddish yellow, group C gray, and group D reddish gray. Shades within each group are based on changes in Chroma and Value, in which increasing Chroma and decreasing Value correspond to higher designated numbers. In principle, shade guides should adequately represent the entire Hue, Value, and Chroma range of natural teeth. However, this is not the case for the VITA Classical shade guide when comparing published tooth color data with published shade guide color data. The VITA Classical shade guide shows deficiencies in RY Hues: Hues extends too far Y, range of Value is too narrow, and Chroma is deficient. How well a guide represents all tooth shades can also be expressed in terms of coverage error. Coverage error is the average color difference between the closest shade guide match and the tooth’s actual/perceived color. The VITA Classical shade guide is organized into groups according to hue, with gradations of Chroma and value within each group.
Shade matching instruments
Colorimeters illuminate a specimen and measure red, green, and blue light reflected back to the instrument. The relative amounts of red, green, and blue are termed the tri-stimulus values, and they define the color of the object. Tri-stimulus values determined by colorimetry do not generally agree with those calculated from spectrophotometric data or those based on the 1931 CIE standard observer and may be affected by the age of the red, green, or blue filters. Colorimeters are commonly used as color difference meters in color production control, which requires a good precision and a high accuracy in color difference measurement. The color evaluation was done using a C-10 PLUS [Minolta, Japan] colorimeter; L*a*b* value was obtained for the above zirconia samples. L* indicates Value. L* = 100 is for white and L* = 0 is for black. a* and b* indicate Hue and Chroma, respectively. *60 and a*-60 indicate red and green, respectively. b*60 and b*-60 indicate orange and red, respectively.
3. Results and Discussion
3.1. Preparation of color shaded zirconia blocks
The color shaded zirconia blocks showed corresponding color matches to A2, A3.5, A4, and B3, respectively. Fig. 3(a) shows the microstructure of the A2, A3.5, and B3 blocks, which were fired in air atmosphere; the blocks fired in reduced atmosphere were denoted as A2N, A3.5N, and B3N, respectively. In Fig. 2, we provide data on the color variation of the prepared zirconia dental block samples, which were treated under atmospheric change using N2 and O2 gas at a higher temperature between 700°C and 1300°C. Fig. 3(b) shows EDS analysis results for the block samples in Fig. 3(a). In spite of the great color change, the EDS results did not show quantitative color element variation because the color pigment addition was under 1 wt%. The valence in transition elements can be easily varied with changes of temperature and atmosphere; a small amount of abrupt valence transition can yield a corresponding color change.11,12, 19–21) It is known that zirconia ceramics maintain a stark white color even at intermediate temperature.5,7) Above 800°C we were able to observe color variation via transition metal element addition of such metals as Fe and Co. In Fig. 4 the color index for the above block samples was revealed by measuring the CRI values using a Minolta CR-10-PLUS; the data analysis is explained in section 3-3.
(a) FE-SEM micrographs for the A2, A3.5, and B3 samples fired in air, and for the A2N, A3.5N, and B3N samples fired in reduction atmosphere under N2 gas flow. (b) FE-SEM-EDS analysis for B3-O1 and B3-3N, respectively.
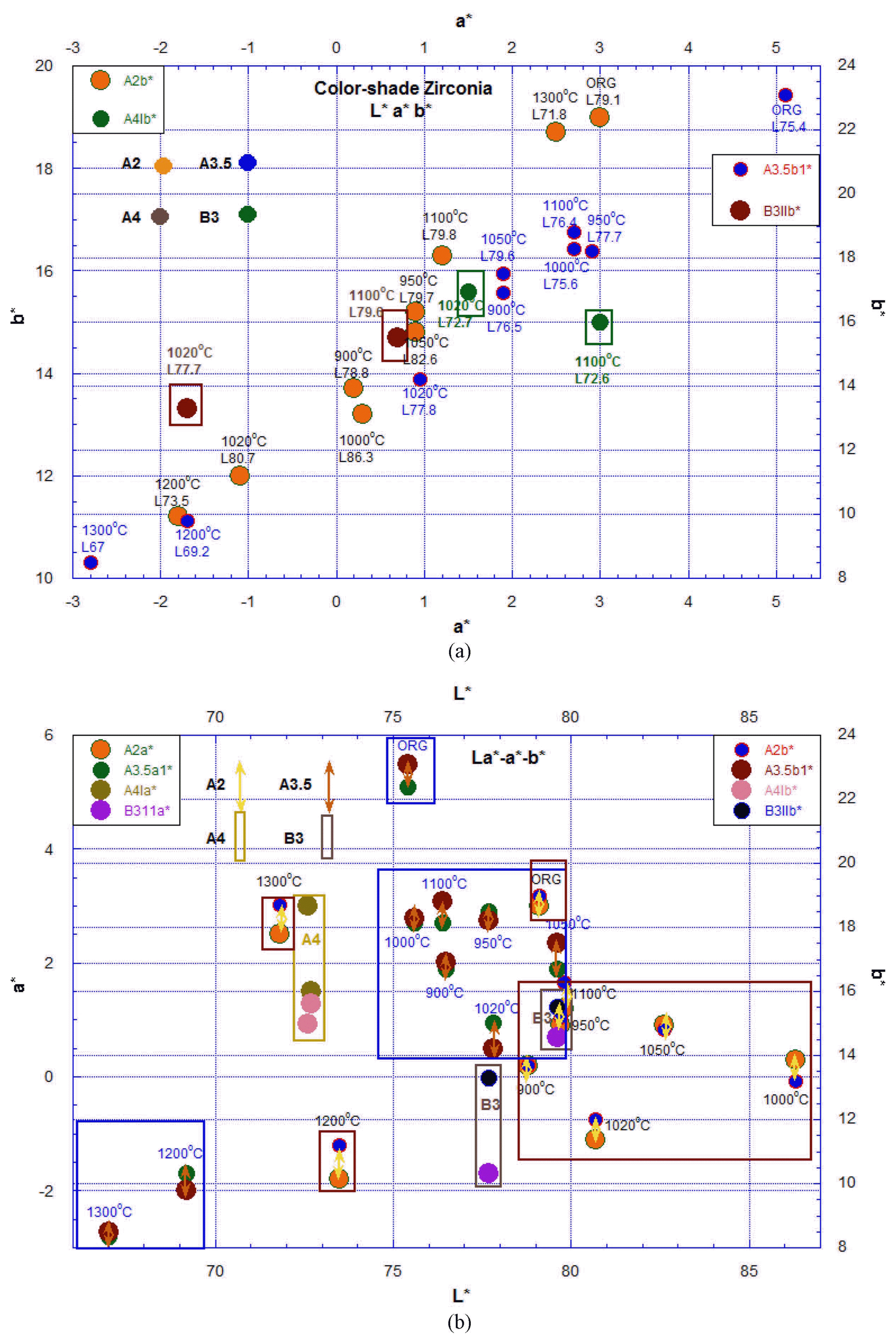
Lab values for the color-shade block samples of A2, A4, A3.5, and B3, which were again fired in O2 and N2 atmosphere. The Lab values were measured by using Colorimeter. (a) L*a*b* (b) La*-a*-b*
In dental block applications it is important to show the translucency after final sintering.8,22–24) In order to impart translucency to zirconia ceramics it is crucial to reduce the levels of oxygen vacancy and impurity, which cause light scattering in the grain boundary according to different localized compositions that have different levels of light reflection. In order to obtain good translucency in 3YSZ dental ceramics, it has been a key issue24) to deplete the oxygen vacancies at the grain boundary misfit region, which leads to an increase of the oxygen concentration. The amount of alumina as sintering additive has been well studied and found to increase the sintering density. In the development of 3YSZ powders for dental blocks, there have been several technological competitions in the areas of reducing oxygen pores in the shaping process, controlling the amount of alumina content, and formulating color additives for tooth color shading.
As a typical coloring agent, the Fe component is homogeneously added through wet chemical precipitation in 3YSZ composition, such as Zpex-Y. The uniform distribution of Fe component in 3YSZ ceramics is important to obtain a uniform color shade. In Zpex-G, the addition of Co component is being performed using the same process. In order to make Erbium stabilized zirconia powders, Zpex-P is prepared by wet chemical method using ZOC [Zirconium Oxychloride] and Er-chloride compound.7,8)
3.2. Atmospheric color change in the sintered zirconia ceramics
Shade matching in human teeth is subjective and challenging because color is not easily quantifiable. It is often difficult to achieve an accurate color match in a dental prosthesis for accurate rendition of patient teeth, which have non-planar surfaces and non-homogenous structure, color, and translucency.
Color is the visual perceptual sensation of light that defines the appearance of our surroundings.23,25–29) Light is the part of the electromagnetic spectrum visible to the human eye. The visible light range includes wavelengths from 380 to 760 nm (CIE 1987). Light photons with shorter wavelengths (400 nm) appear blue and those with longer wavelengths (700 nm) appear red.
Normally in YSZ ceramics it is known that a mixture of Fe2O3 and Co2O3 can yield a coloration element for purple.7,8) Pink is achieved using a polycrystalline zirconia powder stabilized with 8 – 11 wt% Er2O3. All coloring powders with BET 12 – 13 m2/g were prepared using wet chemical method; spray-dried powders with 3 wt% binder were supplied by TOSOH. According to the combination rate (wt.%) in the Table in Fig. 1, we used the formulation batch8,9) for A2, A3.5, A4, and B3. A2 had a formulation batch of 57.85 Zpex-We, 40.0 Zpex-Y, and 2.15 Zpex-P. A3.5 had a formulation batch of 29.03 Zpex-W, 56.67 Zpex-Y, and 4.30 Zpex-P. Additionally, we used B3 batches of 53.18 Zpex-W, 43.33 Zpex-Y, 2.15 Zpex-P, and 1.33 Zpex-G.
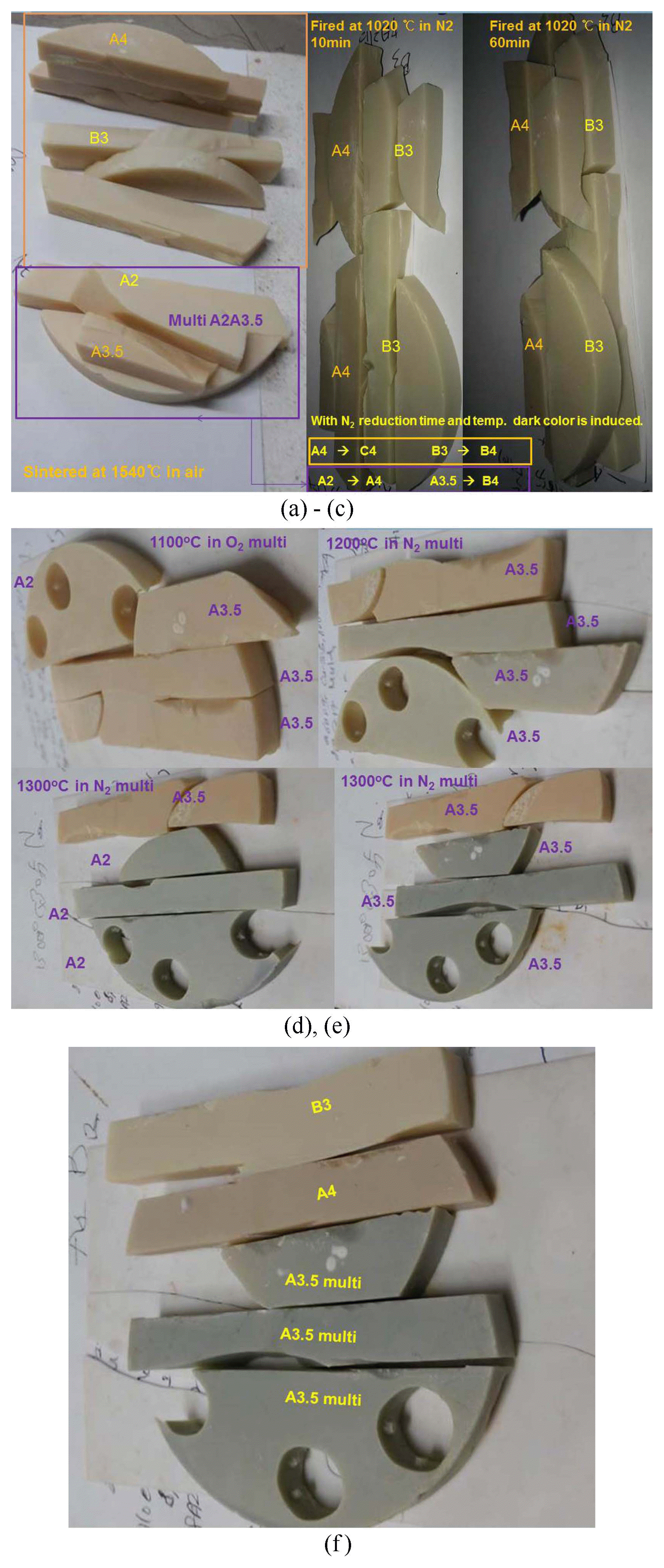
The atmospheric color change during firing is shown in Fig. 4; the measured CRI values were analyzed in section 3-2. The mixing ratio of N2 and O2 was controlled using the pressure controller in a gas bomb. The gas flowed into the plastic tube, and finally was passed through a 100 cc beaker with a flowing speed of two bubbles per second. Fig. 5(a–f) shows the color change for the zirconia block samples, which for PO2 change were atmospherically fired using a mixture gas of N2 and O2. According to the firing schedule of the color blocks, it can be seen that the color variation and the reduction condition, such as the firing temperature and the keeping time, greatly affect the color darkness above 700°C. At above 1300°C there is great color darkness. Fig. 5(a) shows room temperature pictures of A4, B3, and the multi-block, which were sintered at 1530°C for three hours in air. In Fig. 5(b, c) the samples, fired again in N2 atmosphere at 1020°C, show a dark color change. The flow rate of N2 gas is about 300 cc/min. With heating, the N2 gas flow was open at 700°C and maintained from 10 to 120 min at 1020°C. The sample was cooled to 700°C under N2 flow and to room temperature without N2 gas flow. The color darkness increased with heating temperature and keeping time. In the multi-block, the A2 color surface changed to A3 color grade and the A3.5 color surface changed to B4 after passing through the B3 grade. The details of the multi-color block are shown in Fig. 5(d, e). Multi-block was fired at 1300°C in N2 atmosphere. The color change was became dark according to the reduction of the firing temperature and keeping time. The A3.5 surface changed to A4 color grade and the A2.0 surface changed to B4 color after passing through the A3 color grade. In Fig. 5(f) can be seen the final color grade after reduction firing at 1300°C. Samples A4 and B3 are references for color comparison. Sample surface of A3.5 changed to dark A4 color.
3.3. Color index measured using color meter
In ZPex dental ceramics, total light transmission can be attained at 41 – 42% after complete sintering at 1540°C with heating rate of 600°C/h. The total light transmission at 350–600 nm is reduced by the addition of coloring elements such as Fe, Co, and Er in ZPex-Y, ZPex-G, and ZPex-P, respectively. In ZPex-P, Er is an effective element showing a bright red, pink color in zirconia ceramics. Normally, in order to obtain black color in zirconia ceramics, a mixture of transition metal such as Fe, Co, Mn, and Cr can be utilized, but it will result in the loss of light translucency because of the metal oxide mixture, presenting black spots on the zirconia ceramics surface. In ZPex-G, black spots can be observed on the surface of zirconia ceramics due to solid state interaction of Co element with Fe impurity at sintering temperature. In this report we checked the color status in the color shaded zirconia ceramics samples using the VITA classic color scale and a CR-10PLUS color-meter. In reliably matching for tooth shades, visual shade matching is subjective and the results vary among observers and also for an individual observer. The light source influences shade matching because the spectral composition of the light reflecting off an object affects the perceived color as a result of metamerism.30,31) Shade guides are not uniformly distributed in the CIELAB color space32) and do not cover the entire range of natural tooth shades. Finally, enamel translucency and the polychromatic nature of dentin interact to produce depth of shade that is difficult to characterize.
3.4. Raman spectroscopy in color-shade block
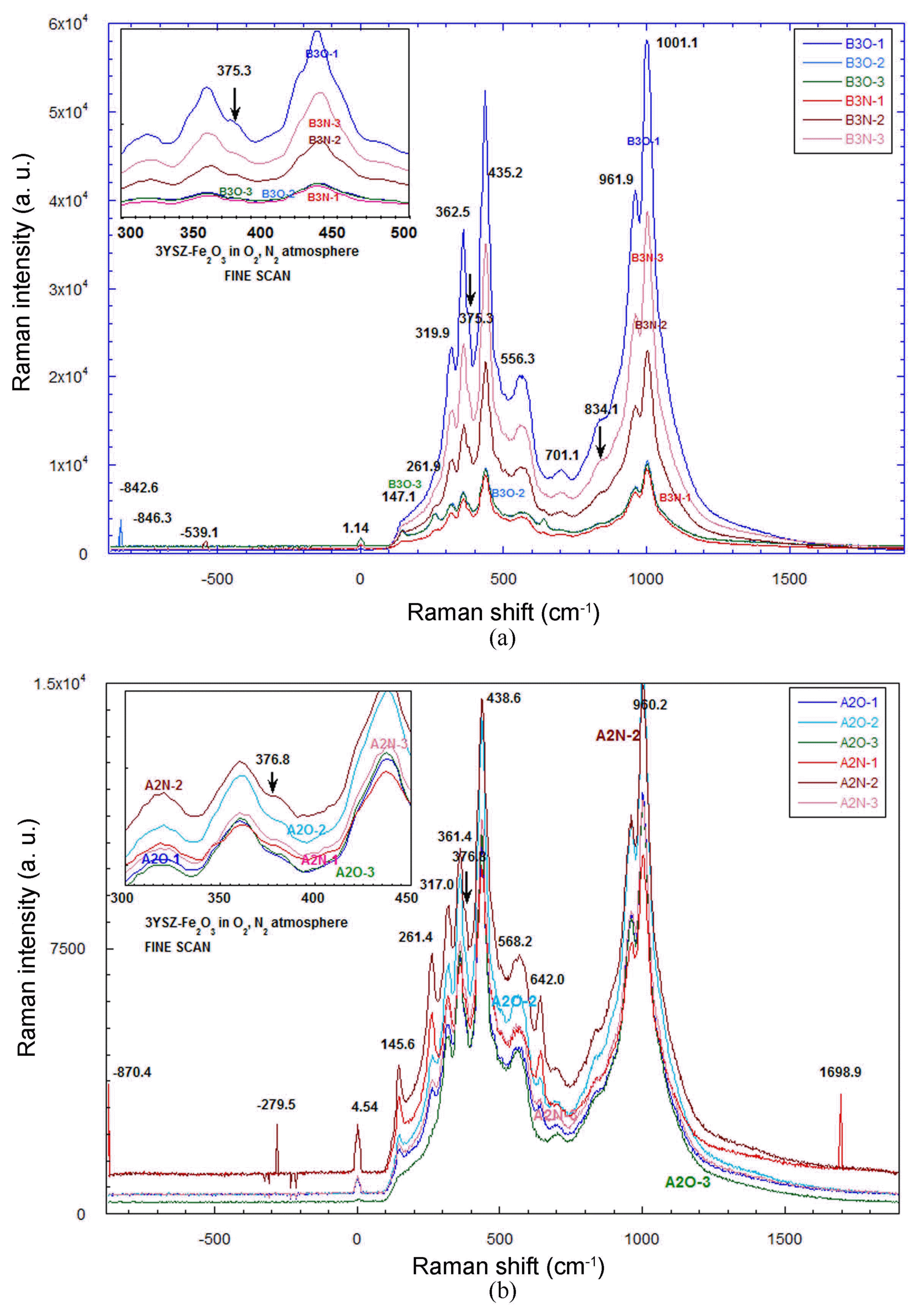
Figure 6 shows Raman spectroscopy results for the color-shade zirconia samples. In Fig. 6(a), it is noted that the Raman band at 834.1 cm−1 shows intensity variation with the change of oxidation and the 375.3 cm−1 band shows slight intensity variation. More oxidized samples such as B3O-1 showed stronger intensity compared to that of the B3N sample treated under N2 gas. In Fig. 6(b) the more oxidized sample of A2O-1 shows a stronger intensity at a Raman shift of 376.8 cm−1. The observed Raman spectroscopy will be further reported through Mössbauer and NMR [Nuclear Magnetic Resonance] measurement, and molecular orbital calculation.33)
4. Conclusions
The color variation in 3YSZ dental color-shade ceramics was investigated by using atmospheric firing using O2 and N2 gas. The color transition was based on the known mixing ratio of coloring elements using Fe, Co, and Er in a 3YSZ matrix. The formulated powders were shaped and sintered at 1530°C. The sintered bodies were fired again under atmospheric condition of O2 and N2 gas at temperature between 700°C and 1350°C with variation of the firing schedule. The obtained samples showed various types of color variation with temperature and firing time. The color was measured using a Colorimeter. We tried to use EDS to analyze the optical color variation effect, without success. Additionally, the measured Raman spectroscopy results showed significant data variation. Additional results will be reported through Mössbauer and NMR measurement, and Molecular Orbital Calcultion.
Acknowledgments
This research was supported by the general research support program of the National Research Foundation (NRF), funded by the Korean Government (NRF-2017R1D1A1B03032397).