Three Dimensionally Ordered Microstructure of Polycrystalline Zirconia Ceramics with Micro-Porosity
Article information
Abstract
In order to make a highly ordered three-dimensionally macro-porous structure of zirconia ceramics, porogen precursors PMMA beads were prepared by emulsion polymerization using acrylic monomer. The monodisperse PMMA latex beads were closely packed by centrifugation as a porogen template for the infiltration of zirconium acetate solution. The mixed compound of PMMA and zirconium acetate was dried. According to the firing schedule, dry compacts of PMMA and zirconium acetate were calcined at 475°C to obtain micro-, macro-, and meso- structures of polycrystalline zirconia with monodispersed porosity. Inorganic frameworks composed of ZrO2 were formed and showed a three Dimensionally Ordered Microstructure [3DOM] of ZrO2 ceramics. The obtained ZrO2 skeleton was calcined at 710°C. The 3DOM ZrO2 skeleton showed color tuning in solutions such as deionized [DI] H2O and/or methanol. The monodispersed crystalline micro-structure with micro/meso porosity was observed by FE-SEM.
1. Introduction
Polycrystalline ceramics are composed of grains and pores. Most of the research on the sintering of polycrystalline ceramics has reported on the behavior of the starting powders and the densification process of the powder compacts. The grains in the ceramics may be close to a single crystal, but the microstructure of an actual grain is a combination of twin crystals. We have focused on the microstructural development of polycrystalline oxide ceramics having uniform grains and monodispersed pores. For structural development, monodispersed oxide powders and porogen beads were prepared using the published methods.1–3) In this study, zirconium acetate solution in methanol as one of the zirconium alkoxide precursors was used to prepare nanocrystalline zirconium dioxide powders. In order to produce mono-disperse pores in the zirconia ceramic matrix we used PMMA [poly(methylmetacrylate)] as a porogen source; PMMA was dried and burned out after mixing with zirconium acetate solution in methanol. The nano-sized crystalline powders of ZrO2 were mono-dispersed.
Three-dimensionally ordered macroporous [3DOM] materials, also known as inverse opals, have attracted attention because of their potential use as photonic crystals operating at visible wavelengths of light. These materials are formed from an artificial opal template, an array of uniform submicrometer polymer, or zirconia spheres. The empty space between the spheres in the template is filled with a metal, ceramic, or semiconductor; then, the template is removed thermally or chemically. The synthesis and properties of 3DOM materials have been extensively reviewed.1–9)
In this research, precursor solution for monodisperse ZrO2 powders was poured into a template of closely packed PMMA. A 3DOM zirconia network was synthesized by colloidal crystal templating.10–14) This nano-porous system can show good benefits such as (1) high surface area originating from its hierarchical nanostructure and (2) a three-dimensionally interconnected porous network facilitating mass transport of reactant molecules.9) The shaped body was dried and fired for the investigation of crystalline phase formation and sintering behavior of the formed ZrO2 phase. Sample bodies calcined at both 475°C and 710°C showed a nano-dispersed honeycomb skeleton of zirconia particles.
2. Experimental Procedure
2.1 Preparation of porogen beads of PMMA
PMMA spheres of uniform diameter were prepared by surfactant-free emulsion polymerization, as shown in Fig. 1(a). Polymerization is initiated with potassium persulfate (for anionically functionalized spheres) or 2,2′-azobis (2-methylpropionamidine) dihydrochloride (for cationically functionalized spheres).1,9) The colloidal suspension was centrifuged to sediment the spheres; then, the solid was dried for at least 24 h at room temperature. A five neck round-bottom flask [Sigma Aldrich, USA] was completely cleaned before the start of the polymer reaction of PMMA. The capacity of the five-neck flask was 3,000 mL; it had a center Joint: ST/NS 24/40. The Cleaning of the PMMA chunks was done using acetone and THF. A flow meter and baking soda were used to remove Cl gas (Fig. 1(a)).
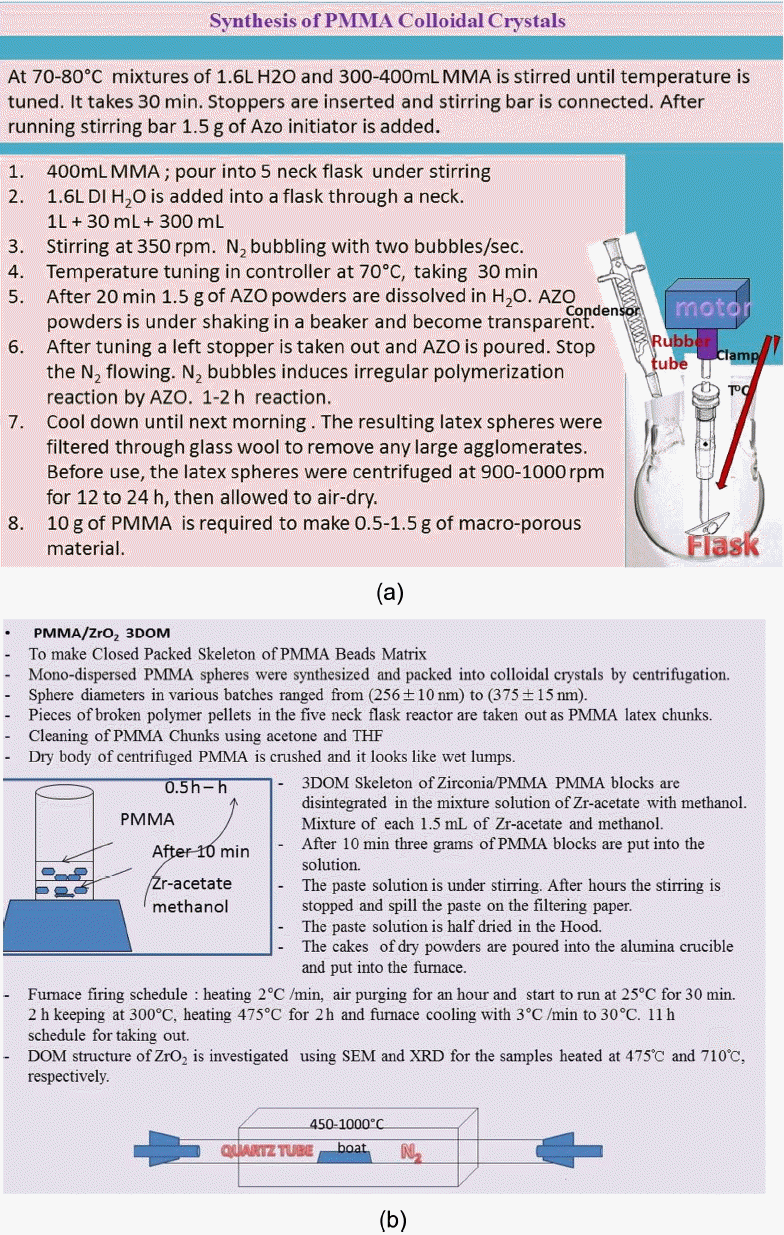
(a) Synthesis of PMMA colloidal crystals and (b) preparation process of 3DOM structured PMMA/ZrO2. Firing furnace for the 3DOMm structure of ZrO2. The firing of PMMA/Zr-acetate was conducted at 475°C and 710°C.
The preparation process of the PMMA beads is illustrated in Fig. 1(a). PMMA spheres were synthesized at 70 – 80°C from mixtures with a typical composition of 1.6 L of water, 400 mL of methyl methacrylate [Methyl methacrylate monomer, M440914 Sigma Aldrich], and 1.5 g of 2,2′-azobis(2-methylpropionamidine) dihydrochloride (AMPD, 97%, Sigma Aldrich) as an azo initiator. Water and MMA were added to the five-neck round-bottom flask, to which was attached an electric stirrer driving a glass stirring shaft [Sigma Aldrich] with a Teflon stirrer blade [Sigma-Aldrich], a water-cooled condenser, a pipet connected to a house supply of nitrogen gas, and a thermocouple probe [Sigma-Aldrich] attached to a temperature controller.
The mixture was stirred at approximately 350 rpm while being heated to 70 – 80°C and purged with nitrogen gas. After stabilization of the temperature at an elevated level, 1.5 g of azo initiator was added and the reaction was allowed to proceed for 1–2 h, producing colloidal PMMA spheres. The colloidal polymer was filtered through glass wool to remove any large agglomerates. PMMA colloidal crystals were formed by centrifuging the colloid at 1500 rpm for 24 h, decanting the water, and allowing the solid to be kept dry for 3 days.
2.2 Preparation of 3DOM skeleton
The construction of three dimensional structures has been difficult and expensive. The chemical construction route involves the use of colloidal crystal templating (Fig. 1(b)) to form 3DOM polycrystalline structures. Monodisperse spheres of PMMA are closely packed into ordered arrays and infiltrated with a fluid that is then solidified. After removal of the template, a solid skeleton is obtained around the ordered array of voids where the original spheres were located. 3DOM structures meet several of the requirements for Photonic Band Gap [PBG] materials: they can be prepared in desirable structures (FCC) with periodicities on a length scale overlapping the wavelength of light, they have the low volume fractions of solid material, and they have a wide range of compositions, which allows modification of the refractive index.10–14)
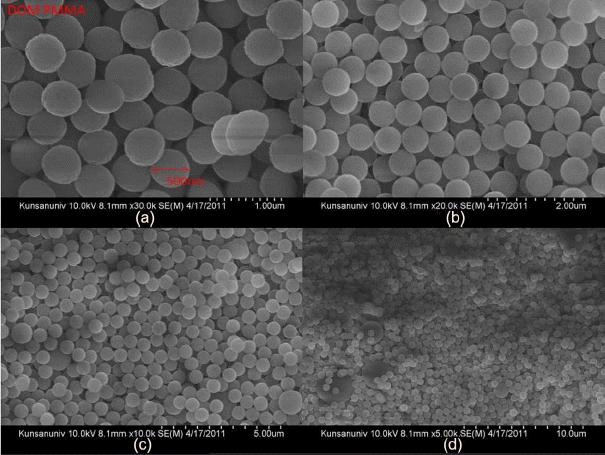
As can be seen in Fig. 1(a), mono-dispersed sized PMMA latex spheres were synthesized in a size range of 500 nm. Using pieces of broken polymer pallets in the flask inner surface, a colloidal crystal template of PMMA spheres was prepared in a centrifuge at 1000 rpm. After polymerization in the five neck flask of the PMMA template, the diameters of the pieces of the broken polymer pellets, which were taken out as types of latex chunks, were between 0.5 cm and 1.5 cm. Dry body of centrifuged PMMA was crushed and looked like wet lumps.
For the synthesis of 3DOM ZrO2, as shown in Fig. 1(b), 1.5 mL of zirconium acetate [Sigma Aldrich] in dilute acetic acid [Sigma Aldrich] was mixed with 1.5 mL of methanol and stirred for 10 minutes. Cationic PMMA beads were soaked in this mixture solution. Three grams of PMMA blocks were put into the mixture solution under stirring. After several hours, the formed paste solution was spilled on a piece of filter paper placed in a Bűchner funnel. Suction was applied to the composite for 10 min to remove excess liquid and remaining compound was dried at room temperature overnight in the hood. The cakes of dry powder were poured into an alumina crucible and put into the furnace, as shown in the last part of Fig. 1(b). According to the firing schedule, the cakes were calcined at 475°C and cooled down to 30°C. In order to investigate the crystal phase change, the calcined sample was fired at 710°C for 2 h. The 3DOM samples calcined at 475°C and 710°C were denoted 3DOM-ZrO2-475 and 3DOM-ZrO2-710, respectively.
A critical factor in optimizing the optical properties of Zirconia materials is the ability to control the fine structure of the walls.15) Zirconia walls are composed of condensed nanocrystals whose sizes influence the optical response of the material. For example, well-ordered 3DOM zirconia with 30 nm wall grains appears white, while a material with 2 nm grains exhibits brilliant colors like opal.14–16) One aspect of our research involves optimizing the nano-grain sizes and phases by controlling the precursor chemistry, template–precursor interfacial interactions, and processing conditions.
2.4 Characterization
XRD (Bruker, M18XCE) was used for the characterization of the dried powders after the firing process (Fig. 2). The microstructure of the sintered ZrO2 body (Fig. 3 – Fig. 7) was investigated using FE-SEM.
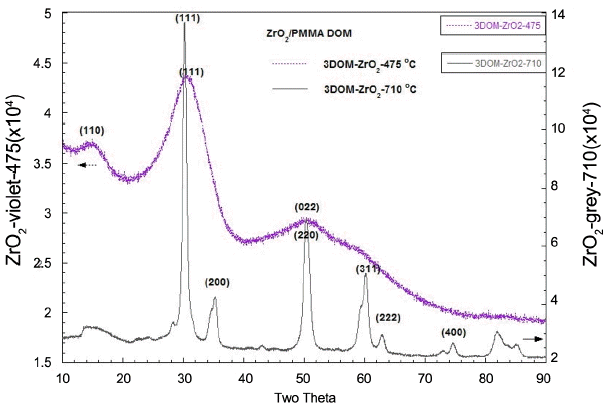
XRD analysis of 3DOM-ZrO2 samples that were calcined at 475°C and 710°C. 3DOM-ZrO2-475 shows a diffused spectra for nano-crystallites; XRD shows a mixture of monoclinic and tetragonal phases. 3DOM-ZrO2-710 shows the typical tetragonal phase indexing.
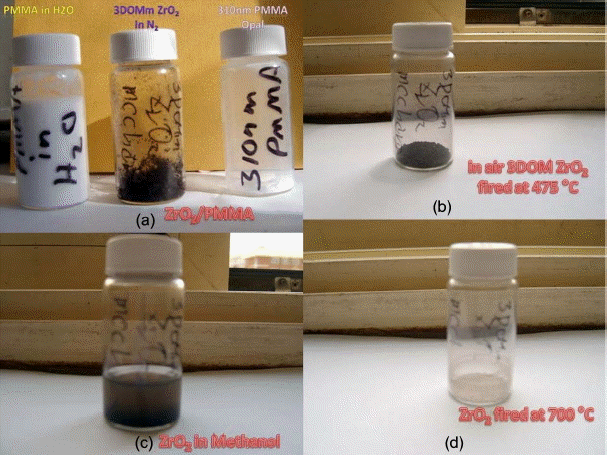
Photographs of the samples of PMMA, 3DOM-ZrO2-475 granules in N2, PMMA in methanol (a), 3DOM-ZrO2-475 in air (b), 3DOM-ZrO2-475 in methanol (c) and (d) 3DOM-ZrO2 powders calcined at 710°C.
3. Results and Discussion
3.1 Preparation of homogeneous beads of PMMA
For the formation of the 3DOM structure of the ZrO2 skeleton, we used PMMA beads as one of the porogen materials; these PMMA beads were prepared by PMMA latex synthesis.1,9) At 70–80°C, mixtures of 1.6 L H2O and 400 mL MMA were stirred until the temperature was tuned, as shown in Fig. 1(a). This process took 30 minutes. Stoppers were inserted into the necks and a stirring bar was connected. Through another neck stopper, N2 gas was flowed under stirring. After 20 minutes of stirring, 1.5 g of AZO powders was dissolved in DI H2O. AZO powders were placed under shaking in a beaker and become transparent. After tuning the operation the one ramining stopper was taken out and AZO solution was poured in. After AZO solution is added into the reactor the N2 gas flow was stopped, because N2 bubbles induce an irregular polymerization reaction with AZO. The next morning, using Glass wool, the polymer was filtered to remove the big coagulates. In order to make 0.5–1.5 g of macroporous material, we used 10 g of PMMA.
PMMA latex spheres with diameters of 500 nm were obtained, as can be seen in Fig. 4. The synthesized raw compounds were stirred and centrifuged in a five neck flask reactor at 1000 rpm. As a colloidal crystal template, pellets were formed from the polymer. Pieces of broken polymer pellets on the surface of the flask surface were taken out; the pellet sizes were from roughly 0.5 cm to 1.5 cm. PMMA is less toxic than polystyrene. For the preparation of the template cakes, the mixture of unreacted PMMA and spheres was softened using acetone and/or THF. Using a spoon, the chunks were pulled and cut. This process was difficult and it took 1.5 h. The obtained PMMA powder aggregates can be ground by just finger pushing the aggregates between weighing covers to grind them.
3.2 Three Dimensionally Ordered Macrostructure of zirconia ceramics
The dry body centrifuged PMMA was crushed and three grams of the PMMA block sample was obtained as wet-like lumps. After 10 min, 3 g of PMMA blocks were put into the Zr-acetate solution and the spheres were disintegrated in methanol. After an hour the zirconium paste was spilled onto the weighing paper and covered. After several hours a paste solution formed under stirring. After an hour the stirring was stopped and the cover was opened. The paste solution was half dried in a hood. The dry powder cakes were poured into the Al2O3 crucible and put into the furnace. The furnace firing schedule was as follows: heating speed of 2°C/min, temperature maintained at 300°C for 2 h, heating and holding at 475°C for 2 h, and cooling 3°C/min to 30°C, as shown in Fig. 1(b). The total firing process took eleven hours to produce the final 3DOM samples of ZrO2.
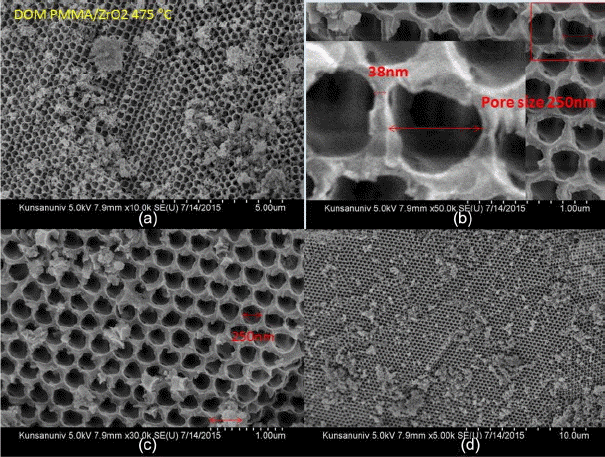
Figure 2 shows the XRD analysis results for the ZrO2 ceramics that were prepared through the calcination of the mixture of Zr-acetate and PMMA beads. From the XRD data, it can be seen that 3DOM-ZrO2-475 has diffused crystal phase spectra, which can be indexed between monoclinic (JCPDS 37-1484) and tetragonal (JPDS 42-1164).17–21) On the other hand, 3DOM-ZrO2-710 clearly shows the tetragonal crystal phase.22) These crystal phase results can be related with the microstructural development of ZrO2 layers shown in Fig. 5 – Fig. 7.
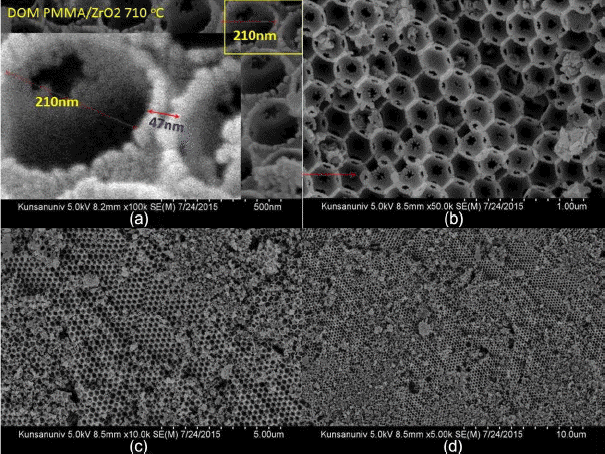
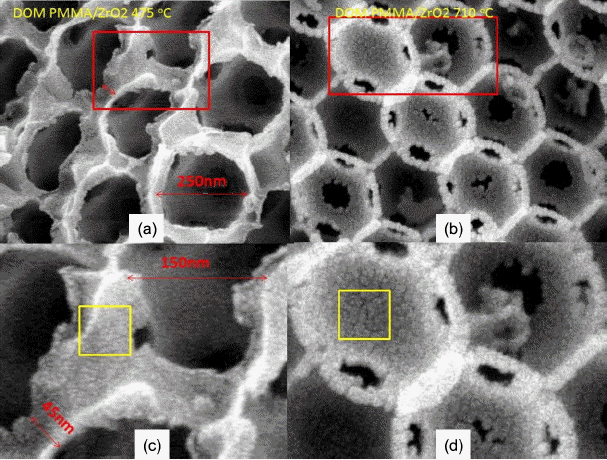
Three pictures in Fig. 3(a) show the PMMA beads in DI H2O, ZrO2 granules calcined at 475°C, and PMMA dry beads, respectively. The PMMA beads are agglomerates of monospheres; the size of the PMMA monospheres is ~ 500 nm, as can be seen in Fig. 4. During the complicated bulk process, mostly monodispersed spheres were formed, and so good building blocks of the monodispersed beads were obtained, as shown in Fig. 4(d). As can be seen in Fig. 4(a) bigger spheres were often observed during the initial stage of condensation and, because of electron emission, irregular shaped beads were observed during the FE-SEM observation. The third picture of PMMA, shown in Fig. 3(a), highlights the opalescent color emission with the light direction, which is the typical behavior of monodispersed beads. The color emission varies with the dimensions of the monodispersed beads and with addition of solutions such as water, alcohol, and toluene.10,14) The second sample of calcined ZrO2 shows a violet color in air (Fig. 3(b)) and a dilute brown color in methanol (Fig. 3(c)). Fig. 3(b) shows the ZrO2 granules calcined at 475°C; the SEM microstructure is shown in Fig. 5. From Fig. 5(c) we can observe the monodispersed pore structure with diameter of ~ 250 nm. From Figs. 5(b), 7(a), and 7(c) the pore size and the boundary thickness of 3DOM-ZrO2-475 were estimated to be ~250 nm and ~38 nm, respectively. In the PMMA/Zr-acetate sample, PMMA was burned out through the slow firing schedule; the formed ZrO2 nano-powders were condensed at 475°C, as shown in Fig. 5. It is supposed from the number of powder grains in the boundary that the diameter of the ZrO2 powder at 475°C can be estimated at ~5 nm. From the 500 nm range of PMMA beads, the pore dimension was found to greatly decrease to 250 nm and 210 nm for DOM-ZrO2-475 and DOM-ZrO2-710, respectively. During the firing process in the formation of the 3DOM skeleton of ZrO2, there were a great burn-out reaction of PMMA beads and a formation with grain growth of ZrO2 nano-powders.
In Fig. 6 it can be seen that the sample was calcined at higher temperature of 710°C and crystallites of ZrO2 condensed into bigger grains in the network boundary region of the PMMA beads. Fig. 7 shows magnified pictures of Fig. 5(b) and Fig. 6(a). The boundary region among the pores, which was formed by the burn-out of the PMMA beads, became larger in the PMMA/ZrO2-710 sample than it was in the PMMA/ZrO2-475 sample. The larger grain size in the boundary region and the smaller pore size of PMMA/ZrO2-715 resulted from densification of the ZrO2 powders.
The next study should try to make progress toward controlling the morphologies of 3DOM materials at different length scales. To prepare periodic pore structures, void spaces between spheres in the colloidal crystal template can be infiltrated by any type of fluid that can penetrate the template and can be converted into a solid. Removal of templating spheres leaves a solid skeleton that surrounds the air holes left in the original locations of the spheres. In the coming report, a yttrium zirconium oxide inverse opal will be made from an artificial opal composed of polystyrene spheres.
4. Conclusions
In order to investigate the three-dimensionally ordered microstructure [3DOM] of ZrO2 ceramics, monodispersed beads of PMMA were prepared through emulsion polymerization using a metacrylate monomer; zirconium acetate was solubilized in methanol. The zirconium acetate solution was immersed in the closely packed skeleton of monodispersed PMMA beads. The mixed dry body of the PMMA and Zr-acetate was slowly fired at 475°C; the fired 3DOM sample of ZrO2 was calcined again at 710°C. The crystal phase and the SEM structure were analyzed by XRD and FESEM, respectively. The 3DOM granules showed an opal-like color transition with light and liquid media such as DI H2O and methanol.
Acknowledgments
This research was supported by the general research support program of the National Research Foundation (NRF), funded by the Korean Government (2013R1A1A2A10058275).