Thermal Evolution of BaO-CuO Flux as Sintering Aid for Proton Conducting Ceramic Fuel Cells
Article information
Abstract
The eutectic melt of BaO-CuO flux is known to be a potential sintering aid for Ba(Zr,Y)O3 (BZY) electrolyte for proton-conducting ceramic fuel cells (PCFCs). A density of BZY higher than 97% of theoretical density can be achieved via sintering at 1300°C for 2 h using a flux composed of 28 mol% BaO and 72 mol% CuO. In the present study, chemical and structural evolution of BaO-CuO flux throughout the sintering process was investigated. An intermediate holding step at 1100°C leads to formation of various impurity compounds such as BaCuO1.977, Ba0.92Cu1.06O2.28 and Cu16O14.15, which exhibit significantly larger unit cell volumes than the matrix. The presence of such secondary compounds with large lattice mismatch can potentially lead to mechanical failure. On the other hand, direct heating to the final sintering temperature produced CuO and Cu2O as secondary phases, whose unit cell volumes are close to that of the matrix. Therefore, the final composition of the flux is strongly affected by the thermal history, and a proper sintering schedule should be used to obtain the desired properties of the final product.
1. Introduction
Proton-conducting ceramic fuel cells (PCFCs) are receiving increasing attention due to their lower operating temperature compared to conventional solid oxide fuel cells (SOFCs);1,2,3,4) Ba(Zr,Y)O3 (BZY) is considered to be a promising electrolyte material for PCFCs due to its inertness towards carbon dioxide and water vapor.5,6,7) One of the major challenges for practical application of BZY electrolyte is its inferior sintering behavior.7,8) A eutectic melt of BaOCuO flux was reported to be an effective sintering aid for BZY9 because the flux, composed of 28 mol% BaO and 72 mol% CuO, melts at 890°C and initiates liquid phase sintering, thereby enhancing densification. In our earlier research work,9) we achieved a relative density up to 97% of the theoretical density at 1300°C with a short soaking time of 2 h by adding 2 wt% of BaO-CuO flux. To achieve such high density without generation of processing defects, optimization of the sintering schedule is important because chemical and structural stabilities are significantly affected by thermal history. In the present study, the effect of sintering profile on the chemical and structural characteristics of the flux was investigated, and a strategy to achieve defect-free BZY electrolyte is presented.
2. Experimental Procedure
For the synthesis of BaO-CuO flux powders, barium nitrate (Ba(NO)3)2, Fluka-Sigma-Aldrich, South Korea), copper nitrate (Cu(NO)3)2.3H2O, Junsei Chemicals Co., Ltd., South Korea) and citric acid (C6H8O7, Sigma Aldrich, South Korea) of A.C.S. reagent grade were used. The nitrate precursors were dissolved in water based on stoichiometry, and citric acid was added in proportion of two moles per mole of metal ion, followed by subsequent addition of ammonia for pH adjustment. The prepared transparent solution was heat treated on a hot plate to transform the solution into a gel, and the viscous gel turned into a solid mass after complete evaporation of water. The solid mass started burning with the heat of the hot plate and, because the reaction is an exothermic one, continued with its own heat along with a small flame.
Before ignition occurred, a hard black mass of BaO-CuO flux was collected and analyzed using differential scanning calorimetry-thermogravimetric analysis (DSC-TGA) at a heating rate of 10°C/min up to 1300°C in air atmosphere.
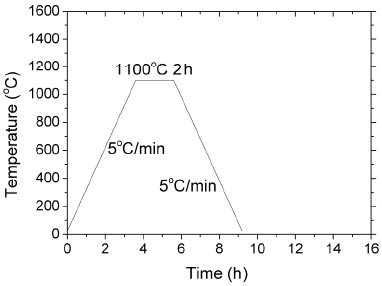
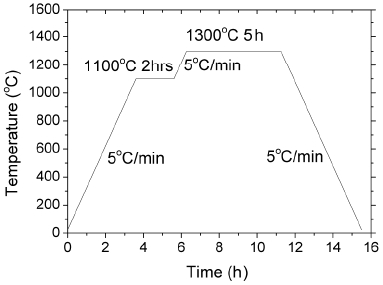
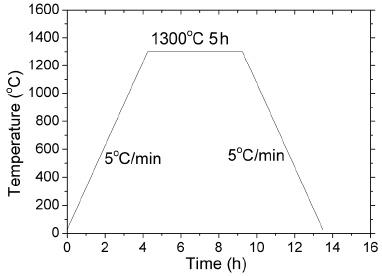
The as-combusted flux was calcined at 700°C for 2 h to get rid of organic residue. The as-calcined flux powder was heated at a rate of 5°C to various temperatures to study its phase formation behavior. A varied heat treatment schedule was employed: heating to 1100°C, holding for 2 h and cooling down (denoted as sample A); heating to 1100°C, holding for 2 h, further heating to 1300°C, holding for 5 h and cooling down (denoted as sample B); and heating directly to 1300°C, holding for 5 h and cooling down (denoted as sample C). Cooling rate was at 5°C/minute for all the experiments. The specifications of these samples are summarized in Table 1. The samples were analyzed using X-ray diffractometry (XRD), X-ray photoelectron spectroscopy (XPS) and scanning electron microscopy (SEM).
3. Results and Discussion
DSC-TGA was carried out for the black gel of the BaOCuO flux to determine the combustion temperature, weight loss profile and melting temperature of flux, as shown in Fig. 1. The measurements were performed between room temperature and 1300°C in air with a heating rate of 10°C/min. The combustion and melting temperatures were found to be 376 and 941°C, respectively. Weight loss was completed during combustion. For powder synthesis, a calcination temperature of 700°C was chosen as an intermediate between the combustion and melting temperatures.
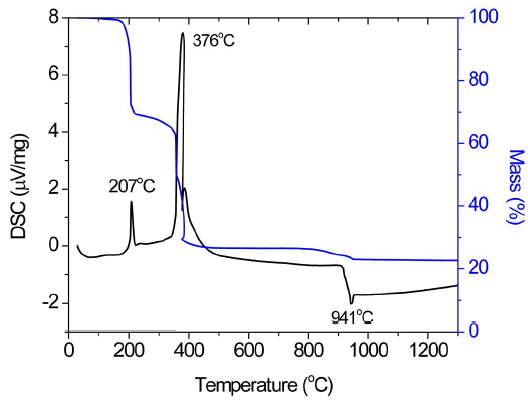
DSC-TGA data of BaO-CuO flux measured between room temperature and 1300°C in air with heating rate of 10°C/min (Fig. 1 is reproduced by permission from Ceramics International 42 (2016) 10476).
Figure 2 shows the XRD patterns of samples A, B and C listed in Table 1. The XRD pattern of sample A, which was held at 1100°C for 2 h, consists of various phases such as cubic BaCuO2 (JCPDS file no.98-000-1049), monoclinic CuO (JCPDS file no. 98-002-6715) and BaCO3 (JCPDS file no. 00-045-1471). BaCuO2, which forms at 1016°C,10) possesses a cubic structure with a unit cell volume of 6098 × 10−6 pm−3. The main peaks of sample B, which was held at 1100°C for 2 h before heating to 1300°C, were indexed to cubic BaCuO1.977 (JCPDS file no. 00-046-0324) and Ba0.92Cu1.06O2.28 (JCPDS file no. 98-004-0587), while the low-intensity peaks indicated the presence of tetragonal Cu16O14.15 (JCPDS file no. 98-000-1381). The cubic and tetragonal compounds possess unit cell volumes of 6121 × 10−6, 6175 × 10−6 pm−3 and 334 × 10−6 pm−3, respectively, while the volume of the mother compound is 74 × 10−6 pm−3 (JCPDS file no. 01-070-3667). Therefore, the unit cell volume mismatch could induce significant residual stresses and, accordingly, mechanical failure. On the other hand, the XRD pattern of sample C, which was directly heated to 1300°C without an intermediate holding step, mostly consists of CuO (JCPDS file no. 98-009-2365) and Cu2O (JCPDS file no. 00-034-1354), along with small amount of monoclinic Ba2Cu3O6 (JCPDS file no. 98-006-3701). Above 1025°C, CuO dissociates into Cu2O, which results in a decreased average copper valence state.11) The unit cell volumes of CuO and Cu2O are 80.9 × 10−6 and 75.0 × 10−6 pm−3, respectively, while Ba2Cu3O6 possesses a unit cell volume of 708 × 10−6 pm−3. The co-existence of these compositions could also cause crack formation; however, in sample C, the amounts of these compounds are relatively small, and mechanical failure can be avoided. Based on the XRD analysis results shown in Fig. 2, it is clear that compounds with large lattice mismatch are readily formed at 1100°C and remain through the subsequent thermal process at 1300°C. In contrast, if the sample is heated directly to the final sintering temperature without intermediate holding, the formation of harmful compounds can be suppressed. Therefore, it is important to use a proper heating schedule to effectively utilize the BaO-CuO flux sintering aid without undesirable side effects.
The valence state of Cu in samples A, B and C was investigated using XPS. The spectra of samples B and C, shown in Fig. 3(a), indicate that a mixture of +1 and +2 states of Cu exists in the compound. Cu has a full 3d orbital in Cu2O, and the peaks detected at 932.7 and 952.09 eV belong to Cu 2p3/2 and Cu 2p1/2 orbitals, respectively.12) The main peak at 932.7 eV was followed by a series of peaks at 933.7 and 940.51. The main peak corresponds to Cu+, and the following two peaks are known as shake-up satellites, indicating a 3d9 shell. Shake-up peaks are generated due to interaction between valence electrons and outgoing photoelectrons, and the relative intensity of main and shake-up peaks can be used to determine the relative amount of Cu+ and Cu2+. In the case of sample B, shown in Fig. 3(a), the intensity of the shake-up peak is close to that of the main peak, implying the presence of a large amount of Cu2+. Contrarily, the amount of Cu2+ is smaller for sample C because the intensity of the shake up peak is lower compared to that of sample B. In Fig. 3(b), it can be seen that the main peak at 933.47 eV, which corresponds to Cu 2p3/2, is followed by satellite peaks at 934.94 and 942.29 eV. The intensity ratio of the main to satellite peaks is less than 1, indicating a higher content of Cu2+ than Cu+. Based on the XRD analysis, it is suggested that the Cu2+ signal arises from CuO, and that Cu+ exists in BaCuO2. The XPS spectra of O 1s were also collected to verify the analysis of the Cu valence state. In general, Cu+-containing samples adsorb oxygen on their surface and generate the peaks for adsorbed oxygen. In the O 1s spectra for sample B (Fig. 3(c)) and sample C (Fig. 3(d)), the dominating peaks are adsorbed oxygen; the presence of lattice oxygen is also evident, which is consistent with a previous report.11)

XPS spectra of (a) Cu for sample B and C, (b) Cu for sample A, (c) O for sample B and (d) O for sample C.
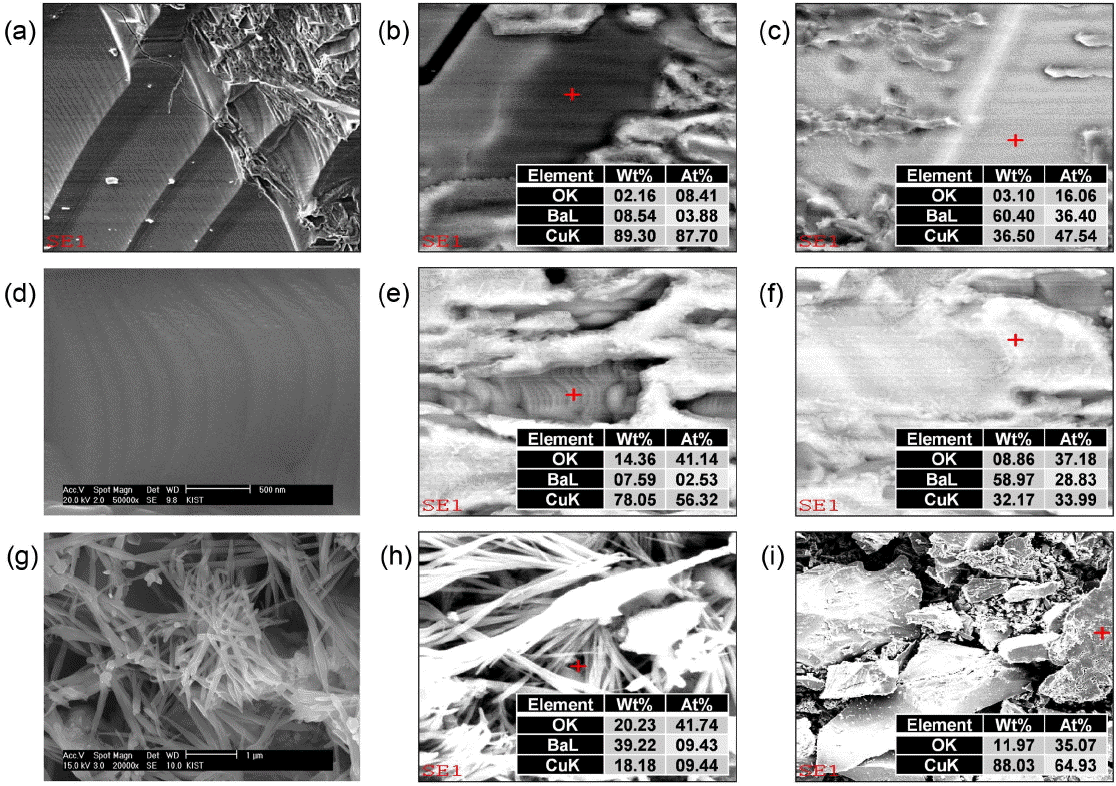
The SEM image of sample B in Fig. 4(a) shows the cleavage planes; EDS analysis of these cleavage planes reveals that the amount of Cu is double the amount of Ba, and two different compositions can be recognized by EDS analysis. The dark area in Fig. 4(b) corresponds to the Cu-rich composition, while the Ba-rich composition appears as a bright area in Fig. 4(c), implying the co-existence of two phases. In Fig. 4(d), cleavage planes are also observed for sample C, but the planes were less sharp compared to those of sample B. For sample C, Ba- and Cu-rich phases were also observed, as can be seen in Fig. 4(e) and (f), respectively, but, based on EDS analysis, their compositions were found to be considerably different from those of sample B. Therefore, it is evident that the holding step at 1100°C significantly affects the evolution of the morphology and phases of BaO-CuO flux. SEM analysis of sample A in Fig. 4(g) and (h) indicates that the growth takes place in the shape of needles at 1100°C; EDS analysis confirmed the presence of BaCuO2, CuO and BaCO3, which is consistent with the XRD results shown in Fig. 2.
4. Conclusions
Thermal evolution of BaO-CuO flux was investigated for potential application as a sintering aid for PCFCs. Three different heating schedules were applied for characterization, and it was found that holding at 1100°C before reaching the sintering temperature leads to formation of BaCuO1.977, Ba0.92Cu1.06O2.28 and Cu16O14.15, which have significantly larger unit cell volumes (6121.66 × 10−6, 6175.83 × 10−6 pm−3 and 334.75 × 10−6 pm−3) compared to that of the mother compound (74 × 10−6 pm−3). Such lattice mismatch can induce large residual stress and mechanical failure after sintering. On the other hand, direct heating to the final sintering temperature without intermediate holding resulted in the formation of CuO and Cu2O, which have unit cell volumes (80.86 ×10−6 and 74.99 × 10−6 pm−3) close to that of the mother compound. Therefore, the final compounds produced after sintering are strongly affected by the thermal history, and mechanical failure can be avoided by using a proper sintering schedule.
Acknowledgments
This research was financially supported by the institutional research program of the Korea Institute of Science and Technology and the Fundamental R&D Program for Core Technology of Materials funded by the Ministry of Trade, Industry and Energy, Republic of Korea (No. 10050985).