Synthesis and Luminescence of Lu3(Al,Si)5(O,N)12:Ce3+ Phosphors
Article information
Abstract
Si4+−N3− was incorporated into Ce3+-doped lutetium aluminum garnet (Lu2.965Ce0.035Al5O12, LuAG:Ce3+) lattices, resulting in the formation of Lu2.965Ce0.035Al5−xSixO12−xNx [(Lu,Ce)AG:xSN]. For x = 0−0.25, the synthesized powders consisted of the LuAG single phase, and the lattice constant decreased owing to the smaller Si4+ ions. However, for x > 0.25, a small amount of unknown impurity phases was observed, and the lattice constant increased. Under 450 nm excitation, the PL spectrum of LuAG:Ce3+ exhibited the green band, peaking at 505 nm. The incorporation of Si4+−N3− into the Al3+−O2− sites of LuAG:Ce3+ led to a red-shift of the emission peak wavelength from 505 to 570 nm with increasing x. Corresponding CIE chromaticity coordinates varied from the green to yellow regions. These behaviors were discussed based on the modification of the 5d1 split levels and crystal field surroundings of Ce3+, which arose from the Ce−(O,N)8 bonds.
1. Introduction
Solid-state lightings (SSLs) have attracted great attention because of their high luminous efficiency, long lifetime, energy-saving potential, etc. One of the prevailing SSL technologies is a phosphor-conversion white light-emitting diode (pc-WLED), which uses a phosphor blend to convert blue or near-ultraviolet light emitted from LED chips into white light. The most popular phosphor materials are nitrides, silicates, aluminates, and phosphates.1–5)
In addition, Ce3+-doped R3Al5O12 garnet systems have been recognized as effective phosphors for use in pc-LEDs combined with blue chips. For example, yttrium aluminum garnet (Y3Al5O12:Ce3+, YAG:Ce3+)6–13) and terbium (Tb3Al5O12: Ce3+, TAG:Ce3+)14) are commercially used as yellow phosphors. Recently, Ce3+-doped lutetium aluminum garnet (Lu3Al5O12:Ce3+, LuAG:Ce3+) was reported to have a value of thermal quenching lower than that of YAG:Ce3+; it is also commercially used as a green phosphor alternative to Eu2+- doped β-sialon for high-power pc-WLEDs.9,15–20)
LuAG has 160 atoms in a cubic cell (space group: Ia3̄d). The Lu and O atoms occupy 24 and 96 sites, respectively, and each Lu atom is coordinated by eight oxygen atoms in a distorted cube. Al atoms occupy two non-equivalent sites, 16 octahedral and 24 tetrahedral.21) The photoluminescence excitation (PLE) spectra of the LuAG:Ce3+ powders consist of strong (~ 450 nm) and weak (~ 345 nm) peaks, which are assigned to the electrons excited from the ground state (4f) doublet to the 5d1 state of the Ce3+ ions.8,19,22) Corresponding photoluminescence (PL) spectra show single emission bands in the green region, which are the result of the two overlapping subbands assigned to the 2D → 2F5/2, 2F7/2 transitions of the Ce3+ ions.15–20)
For the YAG:Ce3+ powders, the effects of the incorporation of Si4+−N3− on the luminescence have been reported in many earlier studies,9–13) whereas there are only a few works in the literature that consider LuAG:Ce3+.9,16) Even though the phase formation and the PL (PLE) spectra of LuAG:Ce3+ doped with Si4+−N3− has been the subject of earlier studies, those studies are still lacking in certain aspects: there is very little information on the phase formation for large amounts of Si4+−N3− or investigation into the PL (PLE) spectra assigned to Ce−(O,N)8 bonds. In this work, we prepared LuAG:Ce3+ powders and investigated the effects of Si4+−N3− incorporation on the phase formation and the luminescence.
2. Experimental Procedure
Lu2.965Ce0.035Al5−xSixO12−xNx [(Lu,Ce)AG:xSN] powders were prepared by a solid-state reaction process using Lu2O3 (99.99%, Solvay), Al2O3 (99.99%, High Purity Chemicals), α-Si3N4 (Ube Industry, Ltd., E10, 99.9%), and CeO2 (99.99%, Grand Chemical & Materials) as starting materials. 2 wt% AlF3 (95%, Junsei) flux was added to the starting mixtures, and the relative amounts of AlF3 and Al2O3 were calculated to maintain a stoichiometric Al content. The starting mixtures were ball-milled for 24 h and synthesized at 1700°C for 7 h under a hydrogen (5% H2 + 95% N2) atmosphere.
The crystal structure was determined using an X-ray diffractometer (XRD, PANalytical, X′Pert3 Powder) with CuKα radiation (λ = 1.5406 Å). Rietveld refinement was performed using X′Pert HighScore Plus (Ver. 4.1). The nitrogen and oxygen contents of the powders were measured using a nitrogen/oxygen analyzer (HORIBA EMGA-920). The PL spectra were measured using a PL system (PSI, Darsa-5000) with a 500 W xenon lamp as an excitation light source.
3. Results and Discussion
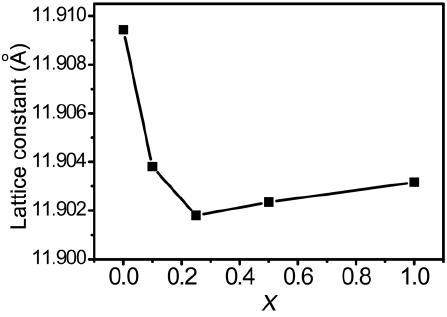
The XRD patterns of the (Lu,Ce)AG:xSN (x = 0, 0.1, 0.25, 0.5, and 1.0) powders are shown in Fig. 1(a). For x = 0–0.25, the patterns correspond to those of ICSD #98-002-3846, indicating that Ce3+, Si4+, and N3− ions were fully incorporated into the LuAG lattices. It was most probable that the Si4+ (r = 0.26 Å for CN = 4, r = 0.4 Å for CN = 6) and N3− ions (r = 1.46 Å) were substituted for the Al3+ (r = 0.39 Å for CN = 4, r = 0.535 Å for CN = 6) and O2− ions (r = 1.38 Å), respectively. As a result, positive
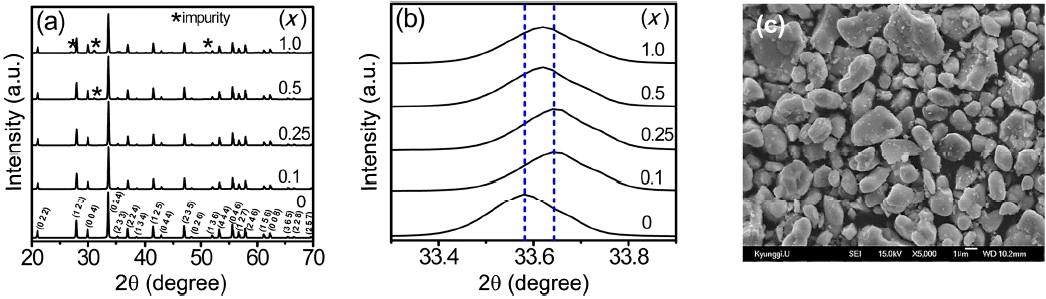
(a) XRD patterns, (b) enlarged XRD patterns of the (Lu,Ce)AG:xSN powders (x = 0, 0.1, 0.25, 0.5, and 1.0), and (c) SEM micrograph for x = 0.25.
The PLE and PL spectra of the LuAG:0.035Ce3+ powders are shown in Fig. 3. The PLE spectrum is composed of two excitation bands, peaking at 345 nm and 450 nm, respectively; these spectra are assigned to the characteristic transition from the ground state (4f) doublet (2F7/2 and 2F5/2) to the excitation levels (5d) of the Ce3+ ions. Under 450 nm excitation, an asymmetrical emission spectrum was obtained in the green region (515 nm); this was the result of two overlapping subbands (dotted lines, centered at 507 and 546 nm, respectively), which can be assigned to the 2D → 2F5/2 and 2D →2F7/2 transitions of the Ce3+ ions, respectively.8,19,22)
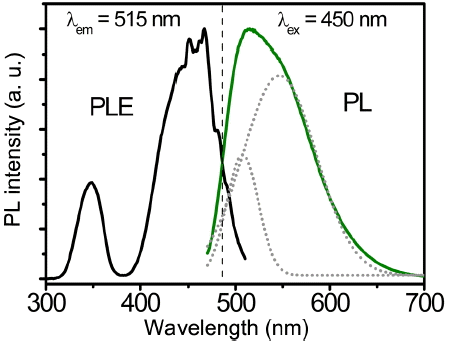
PLE (λem = 515 nm) and PL spectra (λex = 450 nm) of the LuAG:0.035Ce3+ powders. (Dotted lines represent the subbands of the 515 nm emission.)
Normalized PL emission spectra of the (Lu,Ce)AG:xSN powders are shown in Fig. 4(a). With increasing x, the full width at half maximum (FWHM) of the band gradually increases from 96 to 122 nm, and the dominant peak wavelength (DPW) shifts from 515 to 555 nm (red-shift), leading to an increase in the Stokes shift from 2805 to 4204 cm−1. Increasing x values led to a decrease in the emission intensity, as shown in Fig. 4(b). Corresponding CIE (Commission Internationale de l’Éclairage) chromaticity coordinates are shown in Fig. 5. These coordinates move from the green to yellow regions with increasing x; they are (0.3493, 0.5727), (0.3658, 0.5658), (0.3847, 0.5512), (0.3847, 0.5496), and (0.4093, 0.5319) for x = 0, 0.1, 0.25, 0.5, and 1.0, respectively. It is inferred from these findings that the (Lu,Ce)AG:xSN powders have a potential for use in warm pc-WLEDs with high color rendering index (CRI).
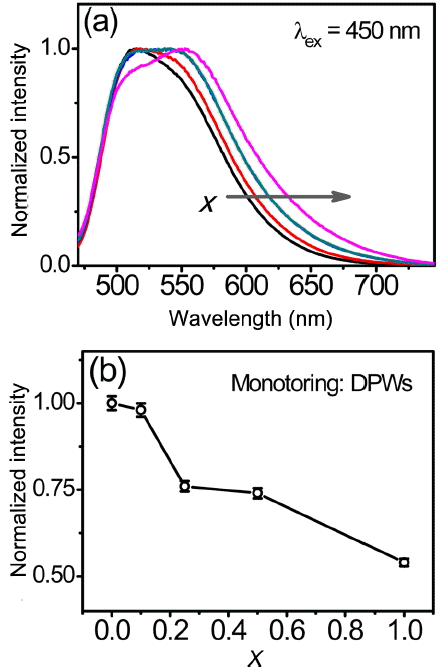
(a) Normalized PL spectra and (b) emission intensity as a function of x of the (Lu,Ce)AG: xSN (x = 0–1.0) powders.
It is obvious that the aforementioned spectral evolution of the (Lu,Ce)AG:xSN powders can be attributed to the incorporation of Si4+−N3− into the Al3+−O2− sites of LuAG. These behaviors can be explained based on those of YAG:Ce3+, because the crystal structures of LuAG and YAG are nearly the same. For the Y3Al5−xSixO12−xNx:Ce3+ powders, earlier works in the literature have described the effects of the incorporation of Si4+−N3− into the YAG:Ce3+ lattices differently. For example, some studies10,12) have reported that nitrogen was not detected in the synthesized powders, despite the addition of Si3N4. As a result, negative defects of
To verify the origin of the asymmetrical broadening and the red-shift of the emission band of the (Lu,Ce)AG:xSN powders, the emission spectrum (λex = 450 nm) of the powders prepared with x = 1.0 was fitted using the Gaussian peak function, as shown in Fig. 6(a). The spectrum was split into three bands of (I), (II), and (III) centered at approximately 501, 545, and 597 nm, respectively. The sum of the fits coincides with the observed spectrum, indicating that this Gaussian fit is reasonable for the emission spectrum. Bands (I) and (II) correspond to two subbands of the emission spectrum of the LuAG:Ce3+ powders prepared without Si3N4 (Fig. 3), revealing that the addition of Si3N4 did not affect the two emission bands of LuAG:Ce3+. On the other hand, the band (III) appears only for the (Lu,Ce)AG:xSN powders. It was inferred from this finding that additional energy levels were created owing to the incorporation of Si4+−N3−. The PLE spectra for the band (III) (597 nm) were measured to determine the origin of the band (III). As a result, in addition to the characteristic excitation peaks at approximately 347 and 450 nm of LuAG:Ce3+, low energy tails were observed in the range of 500–550 nm, and their intensities were found to increase with increasing x values, as shown in Fig. 6(b). The synthesized powders were activated by the wavelength of 520 nm belonging to the low energy tails; the PL spectra are shown in Fig. 6(c). The position and shape of the PL spectra for x = 0.25–1.0 evidently correspond to those of the band (III) in Fig. 6(a). For x = 0.1, the emission intensity at 597 nm rapidly drops; it is not observed for the LuAG:Ce3+ powders (x = 0). It was obvious from these findings that the band (III) and the low energy tail of the PLE spectrum were correlated with each other, and that they can be attributed to the incorporation of Si4+−N3− into the Al3+−O2− sites. The fact that the intensity of the low energy tail increases with increasing x values is a crucial piece of evidence for this speculation. These behaviors coincided with those in the earlier studies for Y3Al5−x SixO12−xNx:Ce3+ 9,11,13) and (Lu,Ce)AG:xSN.9,16) The addition of Si3N4 led to the local substitution of nitrogen ions for oxygen ions, which created two kinds of crystal field surroundings of the Ce3+ ions, Ce–O8 and Ce–(O,N)8 bonds. The latter have higher covalency and polarizability than the former, and the electronegativity of N3− (~ 3.0) is lower than that of O2− (~ 3.4). As a result, the Ce–(O,N)8 bonds enhanced the crystal field strength and lowered the centroid energy of the 5d1 split levels of the Ce3+ ions. The Ce–O8 bonds gave rise to the characteristic PLE and PL spectra of LuAG:Ce3+. On the other hand, the Ce–(O,N)8 bonds caused extra 5d1 split levels of the Ce3+ ions, whose lowest excited level is closer to the ground state than is the lowest excited level of the Ce–O8 bond. This, in turn, resulted in low energy tails of the PLE spectra and the band (III) in the red region. In addition to the effects of local nitridation, it was probable that the substitution of the Si4+ ions for the Al3+ ions also contributed to the red-shift of the DPWs. The incorporation of smaller Si4+ ions distorted the cubic structure of LuAG and increased the degree of the crystal asymmetry, leading to an increase in the crystal field splitting.
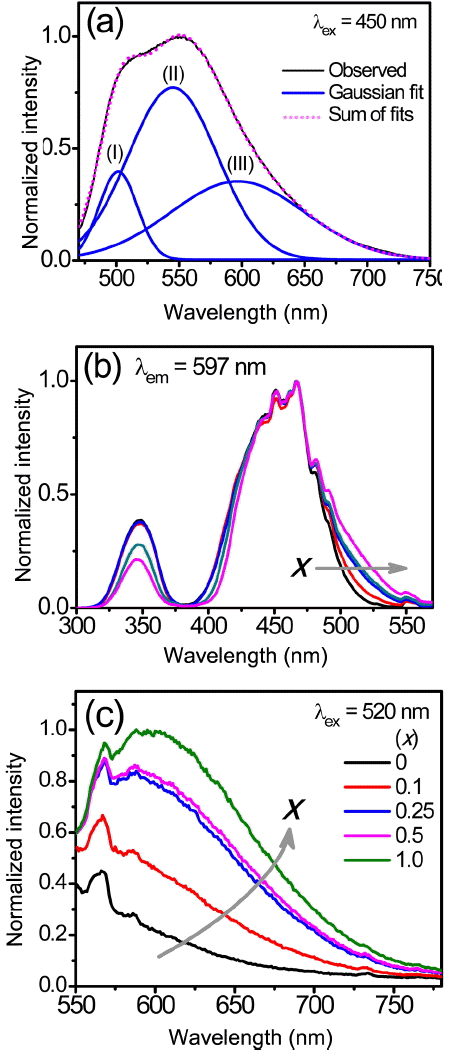
(a) Gaussian fit for the PL spectrum (λex = 450 nm) of the (Lu,Ce)AG:xSN powder prepared with x = 1.0. (b) Normalized PLE spectra (λem = 597 nm) and (c) PL spectra (λex = 520 nm) of the (Lu,Ce)AG:xSN (x = 0–1.0) powders.
Previous works have reported that the thermal stability of LuAG:Ce3+ was very high,16,19) being comparable to nitride phosphors.2,4,5) Liu et al. reported that the incorporation of Si4+−N3− rarely affected the thermal stability up to 150°C, irrespective of the x values.19)
4. Conclusions
(Lu,Ce)AG:xSN powders were prepared with the addition of Si3N4 to LuAG:Ce3+. XRD data confirmed that Si4+−N3− was fully incorporated into the LuAG lattices for x = 0–0.25, resulting in a decrease in the lattice constant because the Si4+ ions were smaller than the Al3+ ions. However, for x > 0.25, the lattice constant slightly increased because of the incorporation of a part of the Si4+ ions into the interstitial sites of LuAG.
The incorporation of Si4+−N3− into the Al3+−O2− sites of (Lu,Ce)AG:xSN resulted in a red-shift of the DPWs and low energy tails of the PLE spectra, leading to a migration of the CIE chromaticity coordinates from the green to yellow regions. These behaviors were attributed to the Ce–(O,N)8 bonds, which modified the 5d1 split levels and crystal field surroundings of the Ce3+ ions. These findings demonstrate that the (Lu,Ce)AG:xSN powders have potential for use in warm pc-WLEDs with high CRI.
Acknowledgements
This work was supported by Kyonggi University Research Grant 2014.