Carbonation Behavior of Lightweight Foamed Concrete Using Coal Fly Ash
Article information
Abstract
The purpose of this study was to prepare lightweight foamed concrete by mixing coal fly ash of circulating fluidized bed combustion( CFBC) with cement, and to develop uses for recycling by analyzing carbonation behavior resulting from a change in conditions for pressurized carbonation. For concrete, CFBC coal fly ash was mixed with Portland cement to the water-binder ratio of 0.5, and aging was applied at room temperature after 3 days of curing at 20°C, RH 60%. For carbonation, temperature was fixed at 60°C and time at 1 h in the use of autoclave. Pressures were controlled to be 5 kgf/cm2 and the supercritical condition of 80 kgf/cm2, and gas compositions were employed as CO2 100% and CO2 15%+N2 85%. In the characteristics of produced lightweight concrete, the characteristics of lightweight foamed concrete resulting from carbonation reaction were affirmed through rate of weight change, carbonation depth test, air permeability, and processing analysis for the day 28 specimen. Based on these results, it is concluded that the present approach could provide a viable method for mass production of eco-friendly lightweight foamed concrete from CFBC coal fly ash stabilized by carbonation.
1. Introduction
Recently, as environment pollution problems due to emission of greenhouse gases along with supply and demand problems resulting from exhaustion of natural coal are emerged, thermal power generation using circulating fluidized bed combustion(CFBC) boilers tends to be increased from the aspects of cost saving by using low-heat coal and of emission regulation for greenhouse gases. However, unlike coal fly ash produced by the existing combustion methods, the coal fly ash produced in the CFBC boilers has a high content of unreacted CaO and low contents of inorganic materials (SiO2, Al2O3, Fe2O3) together with low blaine and powder fineness, failing to conform with the KS specification so as to be buried, thus requiring development of recycling technologies using fluidized bed coal fly ash.
In addition, each countries’ target values for reduction of greenhouse gases were specified through Kyoto protocol as the international treaty for regulation and prevention of global warming. Therefore, since carbon dioxide as one of the object gases is necessarily released into atmosphere upon operation of thermal power plants, solving this problem is important. Mineral carbonation as a technology area for carbon dioxide storage is a technology of conversion to stable carbonate minerals by reaction between minerals and carbon dioxide, and has been developed by Goff1) and Lackner2–4) since an intial study was started in 1990. In the case of coal fly ash produced by the circulating fluidized bed method, chemically stable fixing is possible by reaction with carbon dioxide to form carbonate minerals since large amounts of metal oxides(CaO, MgO) are contained.
In the case of mineral carbonation, it is largely classified into direct, indirect, and other technologies. Direct dry carbonation at a high temperature among direct carbonations involves reaction of fine metal oxides with CO2 gas at a specified temperature and pressure without requiring a separate drying process, making it suitable for an economic process. On the other hand, in the case of direct aqueous carbonation method where carbonation is proceeded by a single process in an aqueous solution, disadvantages are considered to exist since consumption of high costs are caused when applied on a large scale and additional drying processes should be added although it is advantageous to mineral carbonation reactions. Therefore, in the present experiments, pressurized carbonation was conducted at a specified temperature and pressure, and a method suitable for carbonation reaction of the lightweight foamed concrete was selected.5)
When the fly ash was carbonated for recycling as the dry material and the concrete admixture, Kim et al6) stated that there were many advantages such as compactness of concrete matrix structures, improvement in workability as well as long-term strength. Kim et al7) studied the measures for improving mechanical properties of cement matrix by quickening of the reaction for conversion of Ca(OH)2 present inside the matrix to CaCO3 through the use of supercritical carbon dioxide for cement matrix and obtaining the effect of filling fine pores with reaction protubes. While studies on the durability characteristics resulting from carbonation reaction of concrete are being conducted domestically and overseas,8–11) all of them are limited to general concrete matrices. Recently, as the buildings are becoming a high-rise and gigantic, interests in the lightweight foamed concrete capable of reducing self weights are being increased. Also, in the case of steel-reinforced concrete, passive-state films on the surface of steel reinforcement become extinct due to deterioration phenomenon resulting from neutralization, inducing corrosion. Therefore, manufacturing of lightweight foamed concrete is considered suitable for recycling of coal fly ash containing a large amount of CaO produced in the CFBC boilers as well as carbonation of minerals.
Thus, in the present study, the problem-causing CaO when the circulating fluidized bed coal fly ash is recycled as the admixture for cement and concrete was chemically stabilized through direct aqueous hydration and carbonation reaction, and the lightweight foamed concrete with carbon dioxide stored was manufactured through pressurized carbonation reaction. In addition, carbonation behavior of the lightweight foamed concrete as a function of particle size distribution and specific surface area resulting from chemical reactions of coal fly ash together with microstructure and change in contents of carbonation reaction factors was affirmed.
2. Experimental Procedure
2.1 Raw material analysis
For the raw materials employed in the present experiments, ordinary portland cement(OPC, domestic company A) and coal fly ash produced in the CFBC boiler at the domestic thermal power plant Y were used. Chemical compositions of the raw materials were analyzed through XRF (ZSR-100e, Rigaku, Japan), with the results shown in Table 1. In the case of fluidized bed coal fly ash, a high CaO content of 38.35% is shown, most of which exists as unreacted CaO, causing problems of lowered compression strengths, cracking due to expansion, etc. when used as an admixture for cement and concrete. Hence, to solve the chemical instability caused by the unreacted CaO of fluidized bed coal fly ash being contained, hydration and carbonation reactions were implemented according to the following methods.
Through hydration process with agitation after mixing of the fluidized bed coal fly ash and water in the ratio of 1:4, the hydrated fly ash (HFA) was obtained, while the carbonated fly ash (CFA) was obtained for carbonation process by additional injection of CO2 gas in the hydration process at the rate of 1000 cc/min followed by termination of the reaction at the point where the pH became 7. Chemical compositions of thus-obtained hydrated and carbonated fly ashes are shown in Table 1, and it can be affirmed that the hydrated and carbonated coal fly ashes had a large increase in ignition loss as compared with the raw materials.
2.2 Manufacturing method for lightweight foamed concrete
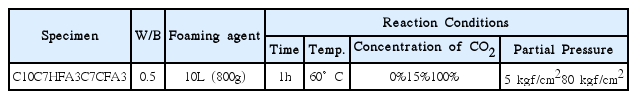
Experiments were conducted with the substituted amount of HFA and CFA for OPC selected as 30 wt.% which showed the most excellent compression strength in the existing experiments. The corresponding abbreviations of each specimen are specified in Table 2, while the mixing and experimental variables upon manufacturing of the lightweight foamed concrete are shown in Table 3. The slurries having undergone hydration and carbonation reactions were mixed with OPC according to each water content after water contents were measured following sedimentation, and the water-binder ratio was fixed at 0.5, and subjected to mixing with the added amount being fixed as 10 L after bubbles were generated by a bubble-generating apparatus using an animal foaming agent. The mixed raw materials were made to fill the circular mold (Ψ100 × 200 mm), and given vibration for a few ten times to prevent the settlement phenomenon. The prepared specimens were then sealed by a zipper bag for 72 h and demolded from the mold after moist curing, followed by aging for 28 days at room temperature and atmospheric pressure.
For the specimen aged for 28 days, carbonation behavior of the lightweight foamed concrete resulting from a change in pressurized carbonation conditions was affirmed by using the autoclave equipment with the pressure and the CO2 gas concentration set as variables. The CO2 concentrations were set for 15% as the exhaust discharge criterion at thermal power plants and 100% as the supercritical condition, and the gas permeation extents of CO2 were affirmed as a function of pressure change. Autoclave(1GNon-Stirred system, Ilshin Autoclave Co. Ltd, Korea) as the carbonation reactor used for the experiments had an O-ring type of sealing, the schematic diagram of which is shown in Fig. 1. For experimental analysis of carbonation extents, the rates of weight change before and after carbonation and the carbonation depths with 1% phenolphthalein sprayed were measured. Also, the air permeability analysis and the pore analysis by using MIP(Mercury Intrusion Porosimetry, AutoporeIII9420, Micromeritics, U.S.A.) were conducted to watch for the changes in pore structures resulting from the carbonation reaction, with additional measurements being made of compression strength(using 50 t universal material tester, Korea).
3. Results and Discussion
To affirm carbonation behavior of the lightweight foamed concrete matrix as a function of substitutional raw materials and changes in carbonation conditions, characteristics of the raw materials, reaction amounts and reaction depths of carbonation were measured, and changes in the pore structures resulting from carbonation reaction were analyzed.
3.1 Characteristics of raw material
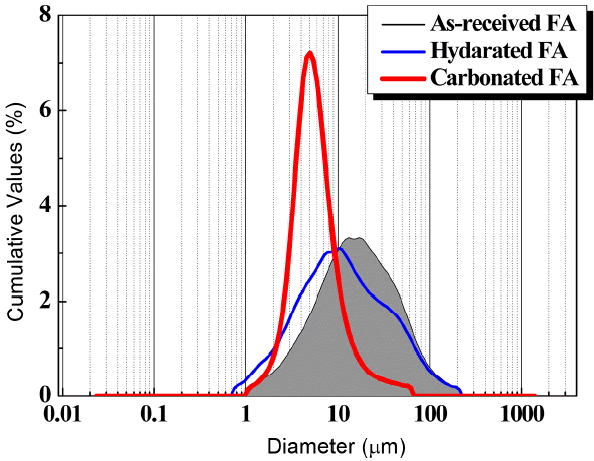
The unreacted CaO as the cause for volume expansion in recycling of fluidized bed coal fly ash upon concrete manufacturing was chemically stabilized through direct aqueous hydration and carbonation processes. The changes in particle size distribution due to phase transformation resulting from the reaction were analyzed by using the particle size analyzer(Sedigraph 5100 CE, Protechkorea, Korea), and specific surface areas were measured by using the air permeability apparatus (DYscale Co. Ltd, Korea). The particle size analyzer is an analysis equipment for measuring the particle sizes according to light scattering by the irradiating laser on the particles. Measurement of specific surface areas was made in accordance with KSL5106(Test method for particle fineness of portland cement by an air permeability apparatus). A particle size analysis graph and the measured values of specific surface area for the circulating fluidized bed coal fly ash used, the HFA and CFA are shown in Figs. 2 and 3, respectively. The further advanced the hydration and carbonation reactions of the as-received fly ash the further refinement results of particles could be obtained. This is considered to be the result produced as the particles were broken by volume expansion through reaction with water and carbon dioxide in the chemical stabilization process of the unreacted CaO.
3.2 Extent of carbonation reaction
Carbonation reaction of concrete is a reaction where CaCO3 and water are generated by the reaction between CO2 and calcium hydroxide (CH), calcium silicate hydrate (CSH) as the CO2 gas is passed through concrete interior, and a reaction affected by factors such as humidity, CO2 concentration, temperature, gas permeability, pressure, etc.
Accordingly, the CO2 concentration and the pressure among the factors affecting the carbonation reaction were set to be variables in the present experiments, causing differences in permeability and pore structure through a change in particle sizes as a function of types of substitutional raw materials. Pressurized carbonation reaction was implemented with the reaction time fixed at 1 h and the temperature at 60°C by using an autoclave after the matrix was cut into the size of Ψ10 cm × 5 cm. At this time, the concentration of CO2 gas was selected to be 15% as the discharge condition for exhaust gases from a thermal power plant and 100% as the collected concentration, and the pressure to be 80 kgf/cm2 for the supercritical condition. Here, to compare the carbonation extents under the atmospheric pressure condition, a low pressure of 5 kgf/cm2 was applied to implement accelerated carbonation, and the rates of weight change along with the carbonation depths were measured as a function of each condition.
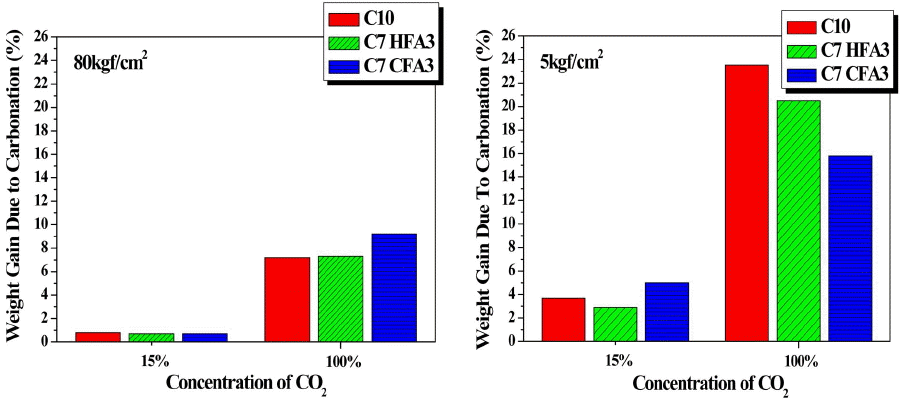
In Fig. 4, the rates of weight change as a function of carbonation conditions are shown for the day 28 matrix. Irrespective of the types of substituted raw materials, the rates of weight change are shown to be increased as the CO2 concentration and the pressure are increased, and the pressures capable of facilitating gas permeation through specimen interior are considered to have a larger effect on the rates of weight change than the CO2 concentration. Under the supercritical condition of PCO2 100% and 80 kgf/cm2, the rates of weight change were increased in the order of 15.79%, 19.05% and 23.53% for the specimens with substitution of CFA and HFA and the C10 specimen without substitution of coal fly ash, respectively. In the case of C10 specimen, this is attributed to the presence of a large amount of unreacted CaO as a factor capable of carbonation reaction. In the case of C7CFA3 as the specimen with substitution of CFA where the unreacted CaO was stabilized by direct aqueous carbonation reaction in advance, only about 70% of carbonation reaction factors existed as compared with C10 specimen, showing a lower rate of weight change in comparison with other specimens. Also, when all reaction factors for carbonation are assumed to react under the supercritical condition, calculation results for the theoretical rates of weight change based on the rate of weight change for C10 specimen (23.53%) were 21.5%(C7HFA3) and 16.8%(C7CFA3), showing the almost same values as the experimental values, and hence allowing affirmation that the extents of carbonation reaction were the same. Park et al12) conducted a study on the supercritical carbonation reaction of general cement by substituting the circulating fluidized bed coal fly ash containing a large amount of carbonation reaction factors for cement, where about 10~13% of weight change rate was observed when the fluidized bed coal fly ash was substituted by 30 wt.%. As compared with C7HFA3 having 21.5% of weight change rate in the present study, formation of tubes within the matrix was possible through bubble injection upon manufacturing of the lightweight foamed concrete, based on which it could be seen that the CO2 permeation through specimen interior was smoother so as to be advantageous to the carbonation reaction.
To affirm the carbonation depths as a function of substituted raw materials and of changes in carbonation conditions, the carbonation extents were affirmed through discoloration due to alkalinity after 1% phenolphthalein solution was sprayed onto the fracture surface of the specimen, with the results being shown in Fig. 5. In the same way as the weight change rates due to carbonation reaction, the carbonation reaction occurred the more smoothly, the more increased were the CO2 concentration and the pressure, and the carbonation depths at the pressure of 5 kgf/cm2 were affirmed to be increased in the order of the specimens without substitution, those with substitution of HFA, and those with substitution of CFA. According to the results of particle size analysis and measurement of specific surface areas, this appears to be the result produced since the more tubes allowing permeation of the gas are formed in comparison with C10 specimen due to occurrence of agglomeration for formation of the more gigantic pores as the more substitution of hydrated and carbonated fluidized bed coal fly ashes having a high specific surface area occurs. In the case of C10 specimen, permeation is considered relatively less as the tubes are narrowed down by the reaction products since a large amount of CaO as the factor capable of reacting with gas was present in the permeation process of CO2 through the specimen interior. Under the pressure of 80 kgf/cm2 for supercritical condition, more than 90% of carbonation can be affirmed to have occurred in all specimens.
3.3 Permeability and pore structure
In general, the pores in concrete are blocked by carbonation reaction, which results in a change in permeability and redistribution of pores, causing a change in the characteristics of gas diffusion into concrete interior. Thus, to affirm the effects of generation of CaCO3 as a reaction product on a change in the pore structure in the lightweight foamed concrete when the pressurized carbonation reaction using an autoclave was implemented, the permeability analysis and MIP(Mercury Intrusion Porosimetry, AutoporeIII9420, Micromeritics, U.S.A.) were conducted.
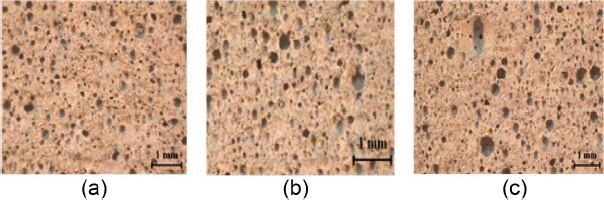
Figure 6 represents the analysis results for permeability before and after pressurized carbonation. Irrespective of specimen types, the tendency for reduction in permeability can be affirmed after the pressurized carbonation occurred. This is considered to be the result of volume expansion due generation of reaction products as the Ca(OH)2 phase is converted to CaCO3 as a result of the carbonation reaction. According to the measurement results of unit volume mass for 3 types of specimens, C10 showed 0.591 g/cm3, C7HFA3 0.69 g/cm3, and C7CFA3 0.68 g/cm3, satisfying the grade 0.6 per KS I 3009(Recycled foamed concrete). Here, the lowest permeability result is observed in C10 specimen having the lowest unit volume mass, which seems to be attributable to the formation of small-sized pores within the specimen and the frequent presence in the form of closed pores. Also, C7CFA3 specimen can be affirmed to have the highest initial permeability before carbonation, which is in agreement with the fact that the reaction occurred in all regions since permeation of CO2 was easiest according to the measurement results for carbonation depths. In Fig. 7, pictures of microstructures are shown for the lightweight foamed concrete manufactured by substitution per raw material. In the C10 specimen which showed the lowest value in the previous analysis results for permeability, frequent presence in the form of closed pores can be seen, and an increase in the pore sizes can be affirmed when the hydrated and carbonated coal fly ashes were substituted. This is the result of agglomeration of fine particles, because of which the carbonation reaction depths are considered to have been increased in the specimens with substitution of the coal fly ash having undergone stabilization treatment.
Figure 8 represents the result of pore distribution obtained through MIP analyses before and after the pressurized carbonation. In the case of pressurized carbonation under the supercritical condition, the size of pores could be seen to be reduced in all specimens. This is considered to be the result of volume expansion as Ca(OH)2 present within the specimen reacted with the CO2 gas injected upon pressurized carbonation reaction to form CaCO3. In the microstructure pictures, formation of the more gigantic pores can be seen as it is changed to C7HFA3 and C7CFA3. This was observed as agglomeration among particles occurred more easily due to an increase in the specific surface area by about 2 ~ 3 times in the case of hydrated and carbonated coal fly ashes. The distribution can be seen to be raised in the pore range of large sizes as may be affirmed in the pore distribution graph before the pressurized carbonation. This means smooth formation of the tubes so as to facilitate gas permeation through the specimen interior, and it could be affirmed according to the measurement results for carbonation depths that the carbonation reaction occurred in all regions due to addition of the carbonated coal fly ash having fine particles.
Also, considering changes in the average pore diameters, a large reduction was observed in the case of C10, suggesting that fine average pore diameters were obtained by the carbonation reaction after pores and tubes relatively larger than those for C7HFA3 and C7CFA3 had existed. On the other hand, a change in the average pore diameters was almost difficult to observe for C7HFA3 and C7CFA3 having a fine particle size distribution and large specific surface areas as they had finer average pore diameters and tubes.
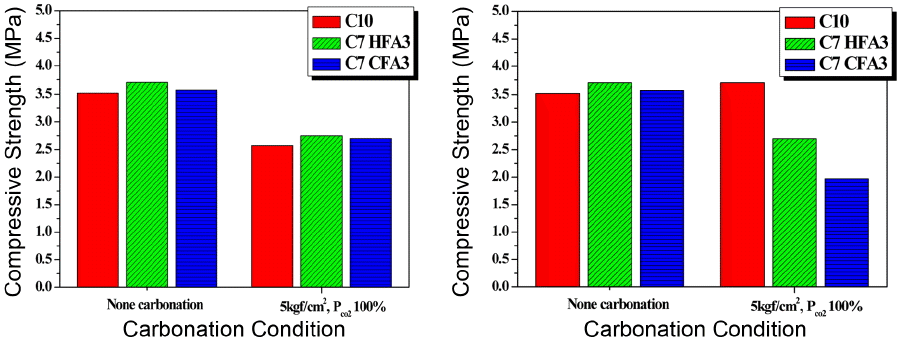
To consider the physical characteristics resulting from a change in pore structures after the carbonation reaction, day 28 compression strength was additionally measured and shown in Fig. 9. Contrary to the expectation where the reaction products would fill up fine pores to increase the compactness of the structure so as to produce favorable effects on the strength as a result of the carbonation reaction, the compression strengths showed reduced results for all specimens after the pressurized carbonation reaction. Unlike the general concrete having strengths higher than 20 MPa, the compression strengths are considered to have been reduced as cracks were developed around pores due to volume expansion as a result of the carbonation reaction in the case of the lightweight foamed concrete having low compression strengths. However, since the lightweight foamed concrete prepared in the present experiment corresponds to the grade 0.6, conforming with KS specification when the strengths higher than 2.0 MPa are indicated, recycling possibilities are considered to exist for the storage of carbon dioxide by using coal fly ash as the concrete admixture.
4. Conclusions
According to the results of affirming the carbonation behavior for the lightweight foamed concrete prepared by stabilization of circulating fluidized bed coal fly ash and substitution for cement by 30 wt.%, the following conclusions have been derived.
By formation of pores inside concrete specimens, a large amount of carbon dioxide could be stored, and the pressure had a greater effect on carbonation reaction than the CO2 concentration, allowing all specimens to store more than 90% of carbon dioxide under the supercritical condition.
When the circulating fluidized bed coal fly ash with stabilization of unreacted CaO was substituted, the contents of carbonation reaction factors were low and the carbonation reaction was relatively less extensive, facilitating gas permeation, and more uniform carbonation reaction was affirmed in comparison with C10 specimen with the carbonation reaction having occurred on the surface.
Through hydration and carbonation reactions, the particle size distribution and the specific surface areas of the raw materials could be changed, as a result of which formation of gigantic pores by agglomeration phenomenon was possible due to the refined particle sizes and overall carbonation reaction was made possible throughout the concrete interior.
According to the results of affirming the carbonation behavior for the lightweight foamed concrete after substitution of the circulating fluidized bed coal fly ash having undergone hydration and carbonation processes, stabilization of the unreacted CaO enabled recycling of coal fly ash as well as manufacturing of the eco-friendly lightweight foamed concrete with carbon dioxide stored.
Acknowledgments
This work was supported by Kyonggi University Research Grant 2014.