Performance of Magnesia Cement Using MgCO3 and Serpentine
Article information
Abstract
The amount of carbon dioxide (CO2) released while producing building materials is substantial and has been targeted as a leading contributor to global climate change. One of the most typical methods of reducing CO2 in building materials is the addition of slag and fly ash, like pozzolan material another method is to reduce CO2 production by developing carbon negative cement. MgO-based cement from the low-temperature calcination of magnesite required less energy and emitted less CO2 than the manufacturing of Portland cements. It is also believed that adding reactive MgO to Portland-pozzolan cements can improve their performance and also increase their capacity to absorb atmospheric CO2. In this study, basic research on magnesia cement using MgCO3 and magnesium silicate ore (serpentine) as the main starting materials, as well as blast furnace slag for the mineral admixture, was carried out for industrial waste material recycling. In order to increase the overall hydration activity, MgCl2 was also added. In the case of the addition of MgCl2 as accelerating admixture, there was a promoting effect on the compressive strength. This was found to be due to the production of needle-like dense Mg-Cl hydrates. Mgnesia cement has a high viscosity due to its high specific surface area therefore, when the PC-based dispersing agent was added at a level of more than 1.0%, it had the effect of improving fluidity. In particular, the addition of MgCl2 in magnesia cement using MgCO3 and magnesium silicate ore (serpentine) as main starting materials led to a lower expansion ratio and an increase in the freeze-thaw resistance finally, the addition of MgCl2 as accelerating admixture led to good overall durability.
1. Introduction
Cement and concrete are the basic substances in construction and civil engineering, and its leading role for the economic growth of our country has been well appreciated. Its production, however, is a highly energy- and resource-consuming one, and emits a large amount of carbon dioxide. Recent awareness on environmental issues drives our concern for energy-saving and environment-friendly methods such as low CO2 emission technologies. It is a global effort to cope with the problem, and our country definitely needs more positive measures on the recycling and sustaining resources, rather than the ordinary ways for material management as presently practiced.
Development of new processes, which are able to provide a drastic reduction of emission is highly required in any fuel-consuming process. In the concrete sector, a strong drive is under way to reduce the amount of cement as much as possible, and to develop a new technology for cementless concrete. At the same time, cement industries worldwide are involved in various methods for the reduction of carbon dioxide; introduction of high-efficiency equipments, utilizing alternate fuels, uses of blended cement, and adopting carbon capture and storage (CCS) techniques.1–6) Domestically, typical studies for reduction of carbon dioxide focus on mixed cement with the addition of pozzolan materials such as slag or fly ash, and on the development of sinterless cement without using any ordinary Portland cement (OPC).
Recently, Novacem in England has developed a new process for so-called carbon negative cements by using low-temperature calcining and sintering of magnesium silicate mineral.7–13) The name of this magnesia-based cement indicates that it can absorb carbon dioxide in atmosphere. The company claims that carbon dioxide absorption during its usage is more than its emission during the production.14–16)
There is no development for such technology domestically. In this study, we involve in manufacturing of cements based on magnesium carbonate and Mg-Si mineral. We add a fine powder of blast furnace slag, and MgCl2 as hydration activators. We evaluate its mechanical property, microstructure by SEM analysis, flow property as a mortar, and durability as a cement and a concrete.
2. Experimental Procedure
2.1 Raw materials
For a preliminary study, we prepared a base magnesia cement with serpentine (xMgO·ySiO2·z(OH)2) and magnesium carbonate (MgCO3) by sintering at proper temperature and time. After increasing its reactivity, the basic hydration properties were evaluated. The magnesia cements with the addition of MgCl2 as a reaction accelerator were also prepared, and its effects on cement property were analyzed.
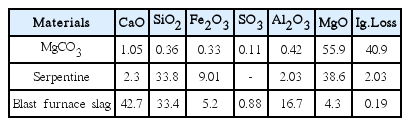
Based on the reported experimental results,17) serpentine was pre-treated with NaHCO3 in an autoclave at 180°C for 5 h to enhance its hydration activity. It was further sintered at 700°C for 1 h and air-cooled. MgCO3 was also sintered at 700°C for 1 h and air-cooled, to have a reactive MgO, followed by milling and mixing with the prepared serpentine for hydration activity. The mixture was added with a fine powder of blast furnace slag, an industrial waste, in an amount of 10 wt% to evaluate its effects on cement reactivity. Finally, the optimum mixture was selected, and MgCl2 solution was added to it to further increase cement reactivity. Table 1 shows chemical compositions of starting raw materials; serpentine, magnesium carbonate, and blast furnace slag.
2.2 Specimen preparation
Reactive magnesium carbonate and serpentine were prepared by treatment in autoclave and sintering, and mixed at 1:1 ratio (MS). The prepared MS sample was added with 10% of blast furnace slag to have MSG sample. The prepared MSG sample was further added with various amounts of MgCl2 (0, 1, 5, 10, 20%) to increase hydration reactivity. Cubic specimen of 2 × 2 × 2 cm were made by mixing the prepared samples with water at water to cement ratio (w/c) of 1, and moist-cured in a constant temperature and humidity chamber (R.H. > 95%) for 1, 3, 7, and 28 days. Mixing compositions of all specimens prepared for this study are shown in Table 2.
2.3 Property evaluation
We evaluated the basic properties such as compressive strength, XRD and SEM for the prepared specimen in terms of hydration time. We followed KSL-ISO 679, cement strength test method, to specify mortar behavior of magnesia cements by measuring their strength and flow property. We also measured stability and durability of the prepared specimen by autoclave expansion ratio test (KS L 5107) for cements and freeze-thaw resistance test (KS F 2456) for concrete.
3. Results and Discussion
3.1 Effects of MgCl2 addition
Figure 1 is a series of XRD results for MS compositions (mixed reactive MgCO3 and serpentine in 1:1 ratio) with various amounts of MgCl2 addition as a hydration accelerator after a 28-day curing. The hydrated phases are mostly Mg(OH)2 and magnesium-silicate hydrates for specimen without or with 5% addition of MgCl2. With 10% Addition of MgCl2, however, peaks from Mg-Cl hydrates start to increase, while Mg(OH)2 peaks decrease. In the case of 20% addition of MgCl2, the generated Mg-Cl-based hydrates become the main phase, and peaks from Mg(OH)2 and magnesium-silicate hydrates are markedly reduced.
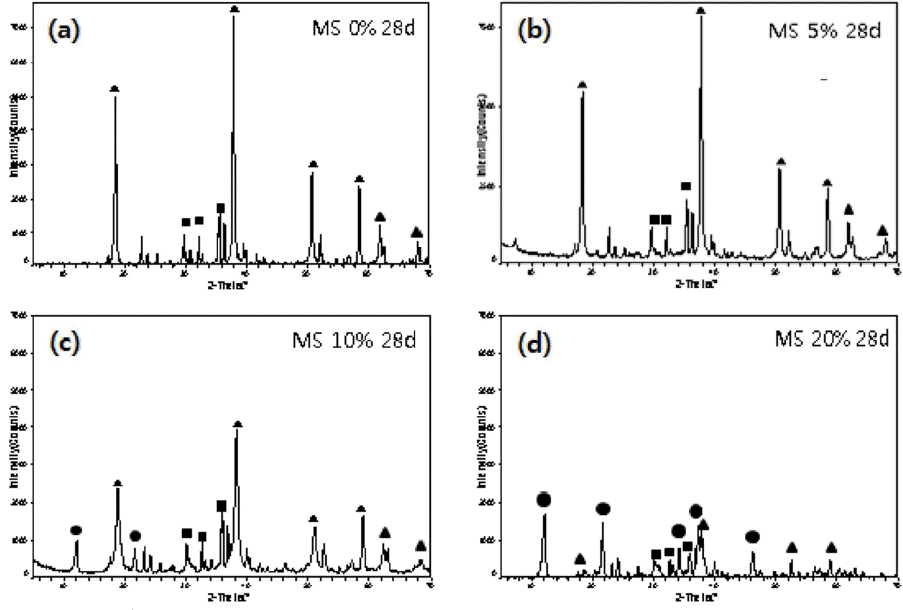
XRD patterns of MS series with different amount of MgCl2. (▲ : Mg(OH)2, ■ : Mg-Si hydrate, ● : Mg-Cl hydroxide hydrate).
Figure 2 is a series of XRD patterns for MSG compositions (mixed reactive MgCO3 and serpentine in 1:1 ratio plus 10% fine powder of blast furnace slag) with various amounts of MgCl2 addition after a 28-day cure. The change of hydrate phases is very similar to the case of MS.
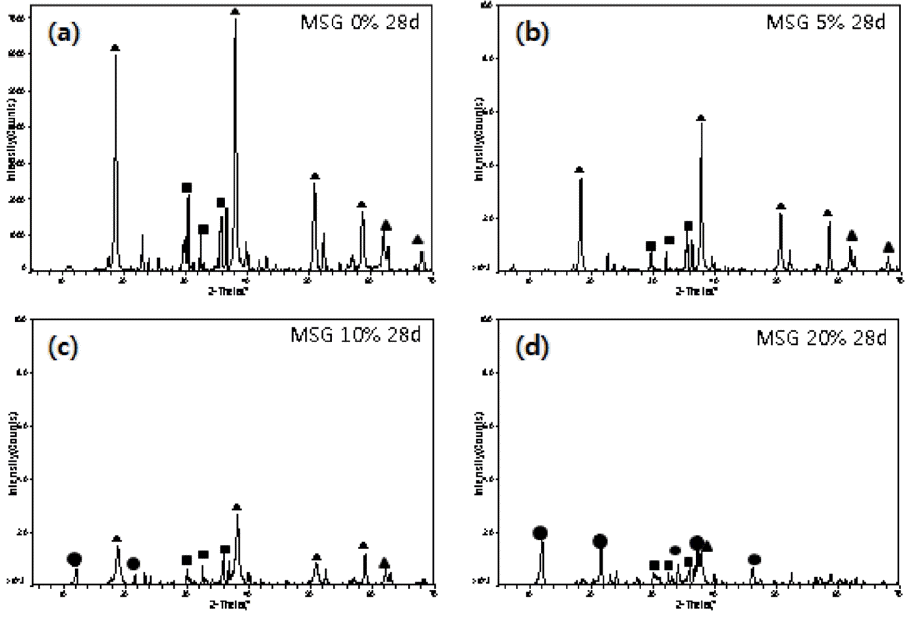
XRD patterns of MSG series with different amount of MgCl2. (▲ : Mg(OH)2, ■ : Mg-Si hydrate, ● : Mg-Cl hydroxide. hydrate).
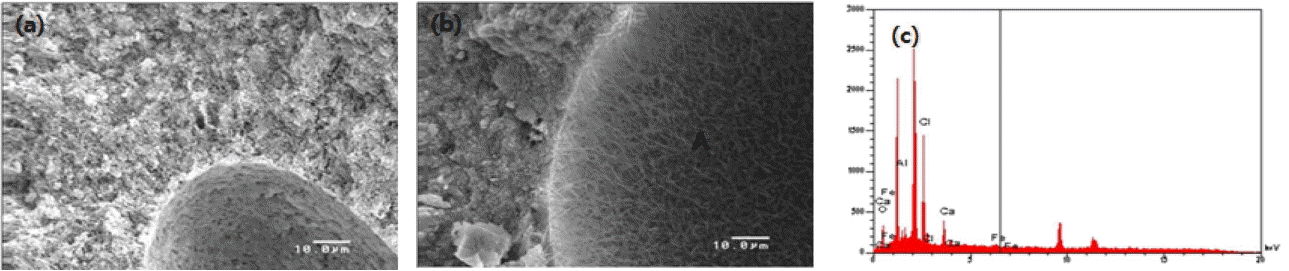
Figure 3(a) and (b) are SEM images with no MgCl2 addition (MS 0) and with 20% MgCl2 addition (MS 20) after 28-day hydration. The latter displays much denser microstructure compared to the former, and shows a formation of needle-like hydrate phase. Fig. 3(c) is EDS analysis of the latter (MS 20), and we find that the needle-like hydrate contains Cl element, which indicates that it is Mg-Cl based hydrate, referring to the XRD analysis in Fig. 1.
3.2 Change of compressive strength with MgCl2 addition
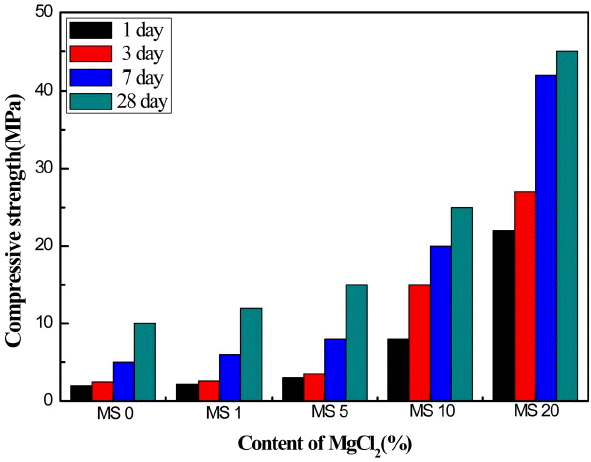
Figure 4 shows compressive strength change for the MS specimen with MgCl2 addition in terms of hydration time. Without MgCl2 addition, compressive strength is only about 2.4 and 10 MPa after 1-day and 28-day hydration, respectively. Compressive strength, however, increases with the increasing amount of MgCl2. For example, With 20% addition of MgCl2, compressive strength is about 22 and 45 MPa after 1-day and 28-day hydration, respectively. This marked improvement on the compressive strength is attributed to the formation of dense needle-like Mg-Cl hydrates as shown in Fig. 1 and 3.
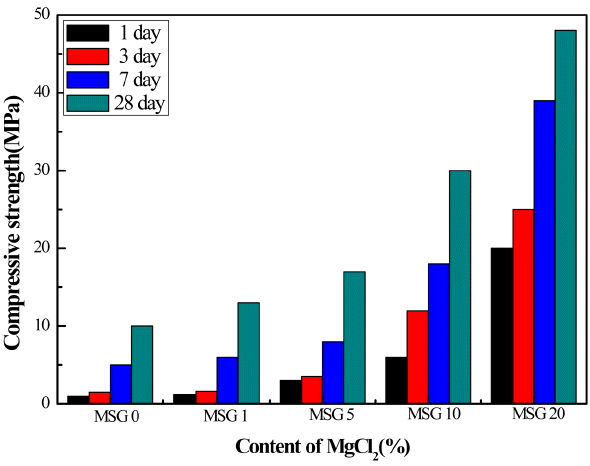
Figure 5 shows compressive strength change for the MSG specimen with MgCl2 addition in terms of hydration time. Without MgCl2 addition, compressive strength is lower and a little higher compared to the MS specimen after 1-day and 28-day hydration, respectively. We observe a similar trend as Fig. 4 with MgCl2 additions. The 28-day strength for the case of 20% addition of MgCl2 is about 48 MPa.
3.3 Mortar behavior of magnesia cements
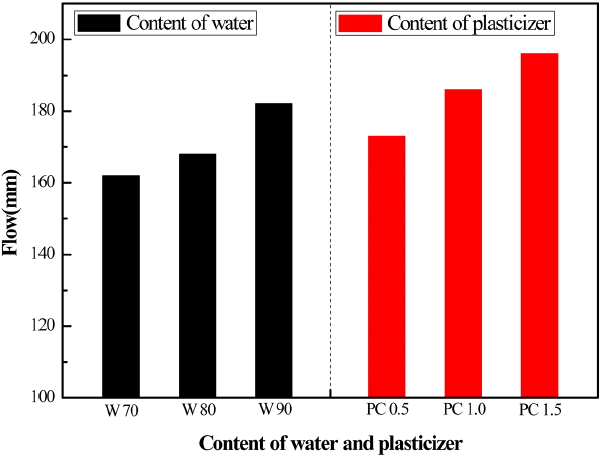
Specimens were prepared as shown in Table 3 to evaluate the MS 20 composition of Table 1 as a mortar. Fig. 6 shows changes in its flow characteristics in terms of w/c ratio and amount of added dispersant. Compared to the ordinary Portland cement (OPC), magnesia cement shows higher viscosity due to the fine particle size, and thus needs more water to have the desired value of flow. Addition of dispersant such as poly carboxyl (PC) improves flow ability. For example, an addition of 1% PC-based dispersant displays a flow of higher than 180 mm at w/c = 0.7.
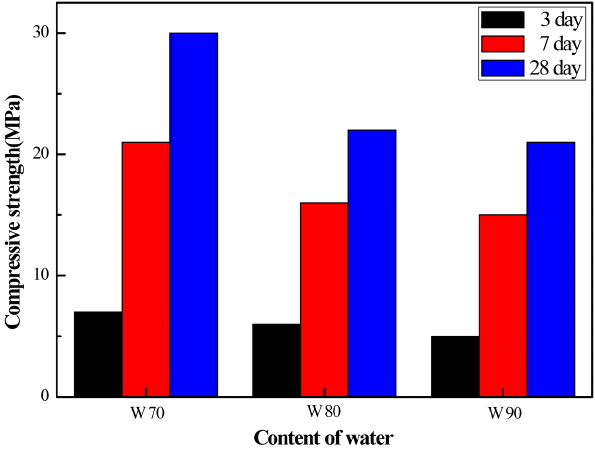
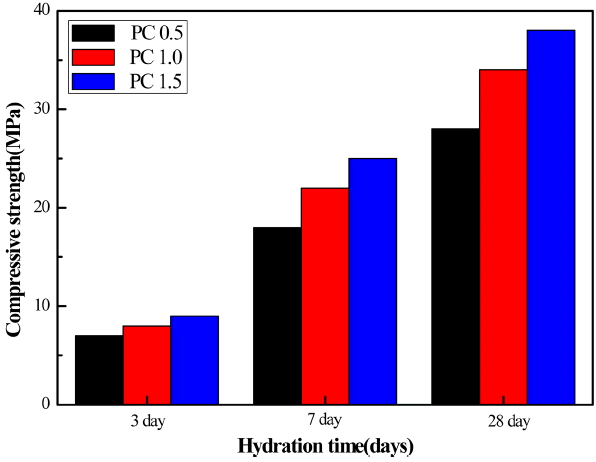
Figure 7 and 8 are changes in compressive strength for MSG 20 specimens as mortars in terms of w/c ratio and in terms of added PC amount at a fixed flow of 160mm, respectively. It is well known that excessive addition of water into either cement or concrete leads to a better flowability but to a reduced strength, as we observe in this study. At fixed flow value, however, increasing addition of PC-based dispersant results in increased compressive strength due to less amount water required.
3.4 Durability evaluation for magnesia cements
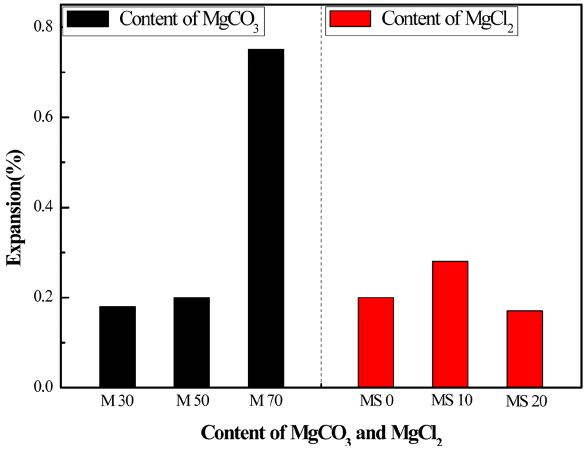
We performed two tests to evaluate durability of the prepared magnesia cements; autoclave expansion-ratio test and freeze-thaw test for concrete made of them. Fig. 9 shows results by autoclave expansion-ratio test according to the KS L 5107 test method. The left figure shows the expansion ratio in terms of MgCO3 and serpentine ratio. Increasing MgCO3 content increases expansion as about 0.20% for 50% MgCO3 and 50% serpentine, and about 0.75% for 70% MgCO3 and 30% serpentine. The right figure shows the expansion ratio for 50% MgCO3 and 50% serpentine, which stays in the stable range of about 0.2% with MgCl2 addition. We thus conclude that the stability of the prepared magnesia cements is affected more by the MgCO3 contents rather than by the amount of MgCl2 addition.
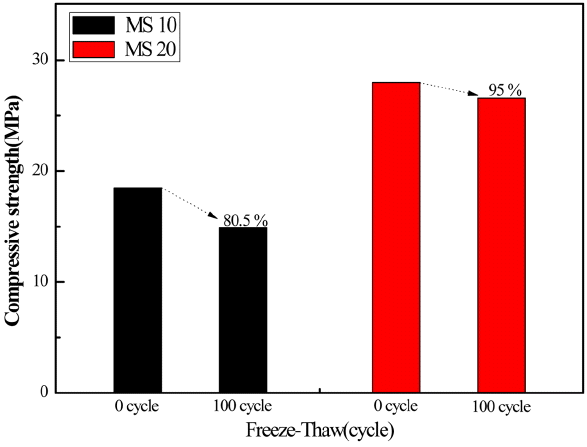
Figure 10 is a summarized result of the freeze-thaw test (KS F 2456) for standard concrete mixtures made of magnesia cements (50% MgCO3, 50% serpentine plus 10 and 20% MgCl2) to evaluate their stability. The compressive strength after 100 cycles of freeze-thaw test is maintained at 80.5% for the case of 10% addition of MgCl2. However, it is much superior 95.0% for the case of 20% addition of MgCl2.
4. Conclusions
We prepare magnesia cements with reactive serpentine and MgCO3, and succeed to achieve an excellent strength by incorporating MgCl2 as a reaction accelerator. The observed enhancement is attributed to the formation of needle-like hydrates at the initial stage of hydration, which makes the structure denser and interlocking. In addition, we find addition of blast furnace slag to the system increases the long-term strength after 28-day curing, although the initial value slightly decreases.
Magnesia cements display relatively higher viscosity due to their high specific surface area. This implies the importance of using of a proper dispersant. And we achieve to reduce w/c ratio and to increase strength by addition of a PC-based dispersant into the prepared magnesia cements.
The prepared magnesia cements exhibit increasing expansion in autoclave test with increasing content of MgCO3. However, we can manage to maintain the value under 0.2% up to 1:1 ratio of serpentine and MgCO3. We further evaluate concrete specimen made of the prepared magnesia cements under freeze-thaw test, and we conclude that they have a good stability when 20% of MgCl2 is added to the system.
Acknowledgments
This study is supported by the research fund (11 Technology Innovation F04) of Construction Technology Innovation Program/Ministry of Land, Infrastructure, and Transport.