1. Introduction
Porous ceramics have been used widely in many areas of industry involving catalyst carriers, membrane supports, filters, and biomaterials.1-3) For these applications, many excellent properties including high porosity and good mechanical strength are typically required.3) Generally, higher porosity tends to result in a certain decrease in mechanical strength.4) Thus, it is important to improve the mechanical strength of porous ceramics with higher porosity, and many studies have been carried out to investigate the relationship between porosity and mechanical strength of porous ceramics.5-9) For example, Chang7) reported that fine Al2O3 grains with a lower degree of agglomeration could increase the bending strength of porous Al2O3 ceramics with a slight increase in porosity. Liu8) found that pores smaller than 2 μm were helpful for improving strength whereas pores larger than 14 μm had the opposite effect. Tang9) developed a routine including directional freeze casting and annealing in a tertiary butyl alcohol (TBA)-water-sucrose system that could significantly improve the compressive strength of porous alumina ceramics. However, until now, the effects of Al(H2PO4)3 addition on the porosity and flexural strength of porous Al2O3 ceramics had not been investigated in detail. In this work, Al(H2PO4)3 was used as an additive to fabricate porous Al2O3 ceramics, and the effects of Al(H2PO4)3 addition on the porosity and flexural strength of the ceramics were studied extensively.
2. Experimental Procedure
Al2O3 powders with purity > 99.0 wt% were used in this work. The average particle size of these powders was about 0.9 μm. The Al2O3 powders were mixed with 0.5 wt% polyvinyl alcohol (PVA) solution (concentration of 5 wt%) and chemically pure Al(H2PO4)3 with additions of 0, 5, 10, 15, and 20 wt%. Then, green rectangular bodies of 5.0 mm × 10.0 mm × 50.0 mm were bidirectionally pressed under the pressure of 35 MPa. After drying at 50°C for 10 h, the green bodies were heated to1300°C, 1350°C, and 1400°C at a heating rate of 5°C/min and then soaked for 2 h in air.
The water absorption and open porosity of the as-prepared Al2O3 ceramics were measured using Archimedes principle. Ignition loss (IL) was calculated by measuring the weight of the bodies before and after sintering. Flexural strength was tested via a three-point bending test (ATOGRAPH AG-I, Shimadzu) with a support distance of 30.0 mm and cross-head speed of 0.50 mm/min. The crystal phase compositions of the sintered Al2O3 ceramics were determined with an X-ray diffractometer (XRD; D8 Advance, Brucker, Germany) using a Ni-filtered Cu Kα radiation source (λ = 0.154178 nm). The microstructures of fracture surfaces of the sintered specimens were characterized with a field emission scanning electron microscope (FESEM; Sirion 2000, FEI, Netherlands). Pore size distribution was determined by mercury porosimetry (AutoPore IV 9510, Micromeritics, USA).
3. Results and Discussion
3.1. XRD
Figure 1 shows the XRD patterns of the 1350°C sintered ceramics with various additions of Al(H2PO4)3. As can be seen from Fig. 1, all peaks in the five patterns can be attributed to the characteristic peaks of corundum (α-Al2O3, JCPD S 46-1212), and no appreciable peaks of Al(PO3)3 or AlPO4 were found, indicating that most of the added Al(H2PO4)3 was eventually decomposed into Al2O3, H2O, and P2O5 during the course of sintering. The chemical reaction can be described as follows:
Thus, it can be inferred that the as-produced H2O and P2O5 in the form of gas evaporated out of the ceramics during sintering and that only solid Al2O3 remained. Therefore, only the characteristic peaks of α-Al2O3 occur in the patterns.
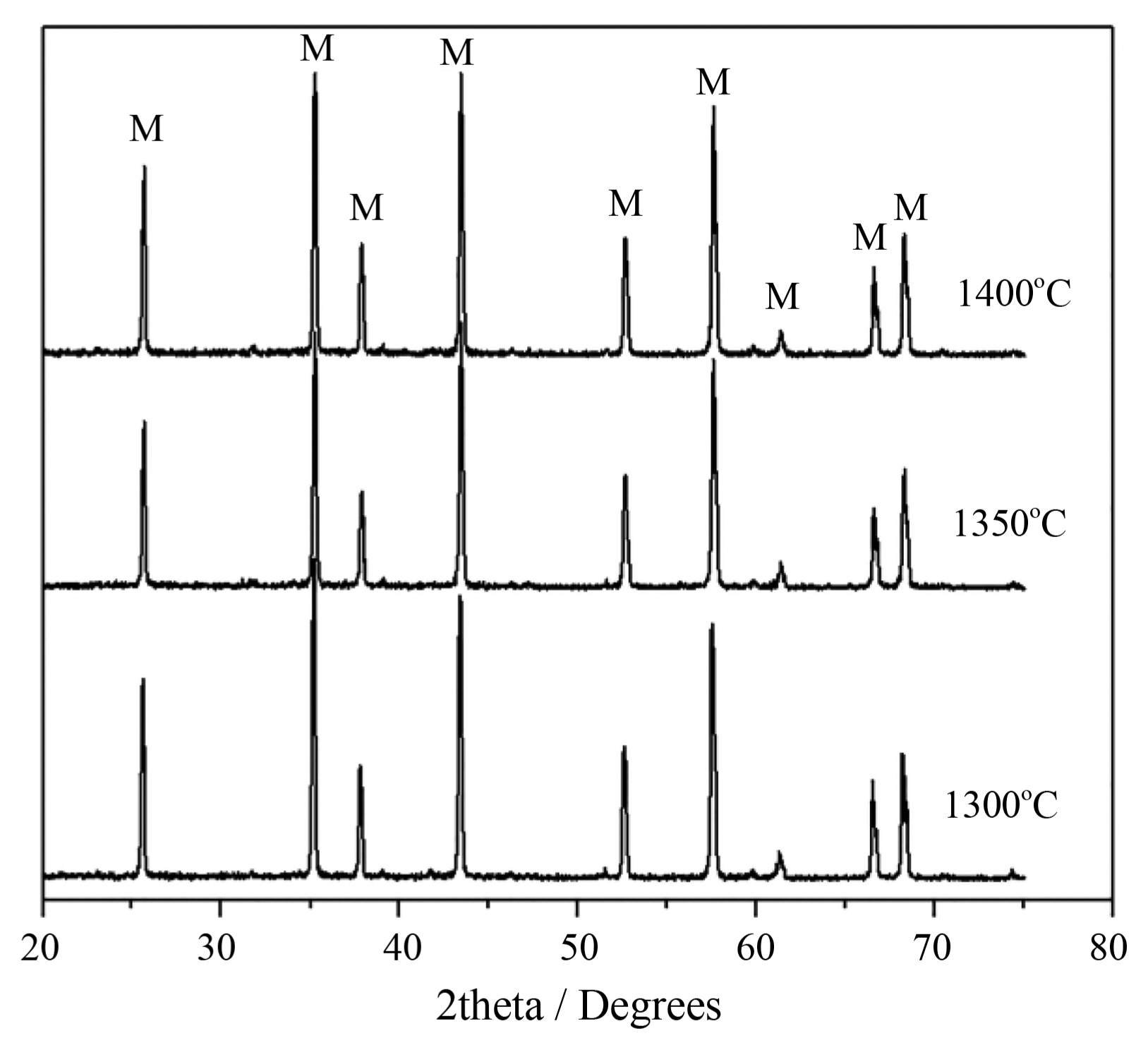
Figure 2 presents XRD patterns of the ceramics with addition of 20 wt% Al(H2PO4)3 sintered at 1300, 1350, and 1400°C. As shown in Fig. 2, only characteristic peaks of corundum (α-Al2O3, JCPD S 46-1212) occur for the ceramics sintered at the three temperatures, revealing that the added Al(H2PO4)3 was eventually decomposed into Al2O3, H2O, and P2O5 during the course of sintering.
3.2. Properties of the porous ceramics
The properties, including water absorption, open porosity, bulk density, and flexural strength, of the as-prepared porous ceramics are summarized in Tables 1, 2, and 3. As shown in the tables, the water absorption, open porosity, and bulk density of the porous Al2O3 ceramics increased remarkably with increasing Al(H2PO4)3 addition from 0 to 20 wt%, indicating that the gaseous H2O and P2O5 resulting from decomposition of the Al(H2PO4)3 increased open porosity. Meanwhile, the flexural strength of the ceramics first increased significantly with increasing Al(H2PO4)3 addition from 0 to 5 wt%, although the open porosity increased remarkably. Additionally, compared to that of the ceramics without Al(H2PO4)3 addition, the porous ceramics with 10 wt% Al(H2PO4)3 addition exhibited no appreciable deterioration in flexural strength, despite the remarkable increase in open porosity. Subsequently, the flexural strength of the porous ceramics decreased slightly with further increase in Al(H2PO4)3 addition. In conclusion, addition of 5-10 wt% Al(H2PO4)3 was significantly advantageous to the flexural strength as well as the open porosity of the porous Al2O3 ceramics sintered at 1350°C.
3.3. SEM characterization
Figure 3 shows the SEM microstructures of fracture surfaces of the porous Al2O3 ceramics with various additions of Al(H2PO4)3 sintered at 1350°C. As revealed by Fig. 2, more pores were observed in the fracture surfaces with increasing addition of Al(H2PO4)3, in good agreement with the experimental results shown in Table 1. Moreover, the average grain size of Al2O3 became appreciably smaller as the Al(H2PO4)3 addition increased from 0 to 5 wt%, which may be, at least partly, responsible for the higher flexural strength of the latter than of the former, because flexural strength was is in proportion to d−1/2 (d: grain size) despite the higher porosity of the former.10) With further Al(H2PO4)3 addition, the porosity and average Al2O3 grain size of the porous ceramics became larger, so the flexural strength became lower. It is worth noting that the ceramics with 10 wt% Al(H2PO4)3 addition exhibited flexural strength comparable to that of the ceramics without Al(H2PO4)3, although the latter had much higher porosity. Thus, it is reasonable to conclude that addition of 5-10 wt% Al(H2PO4)3 can enhance the flexural strength and open porosity of the Al2O3 ceramics.
3.4. Pore size distribution
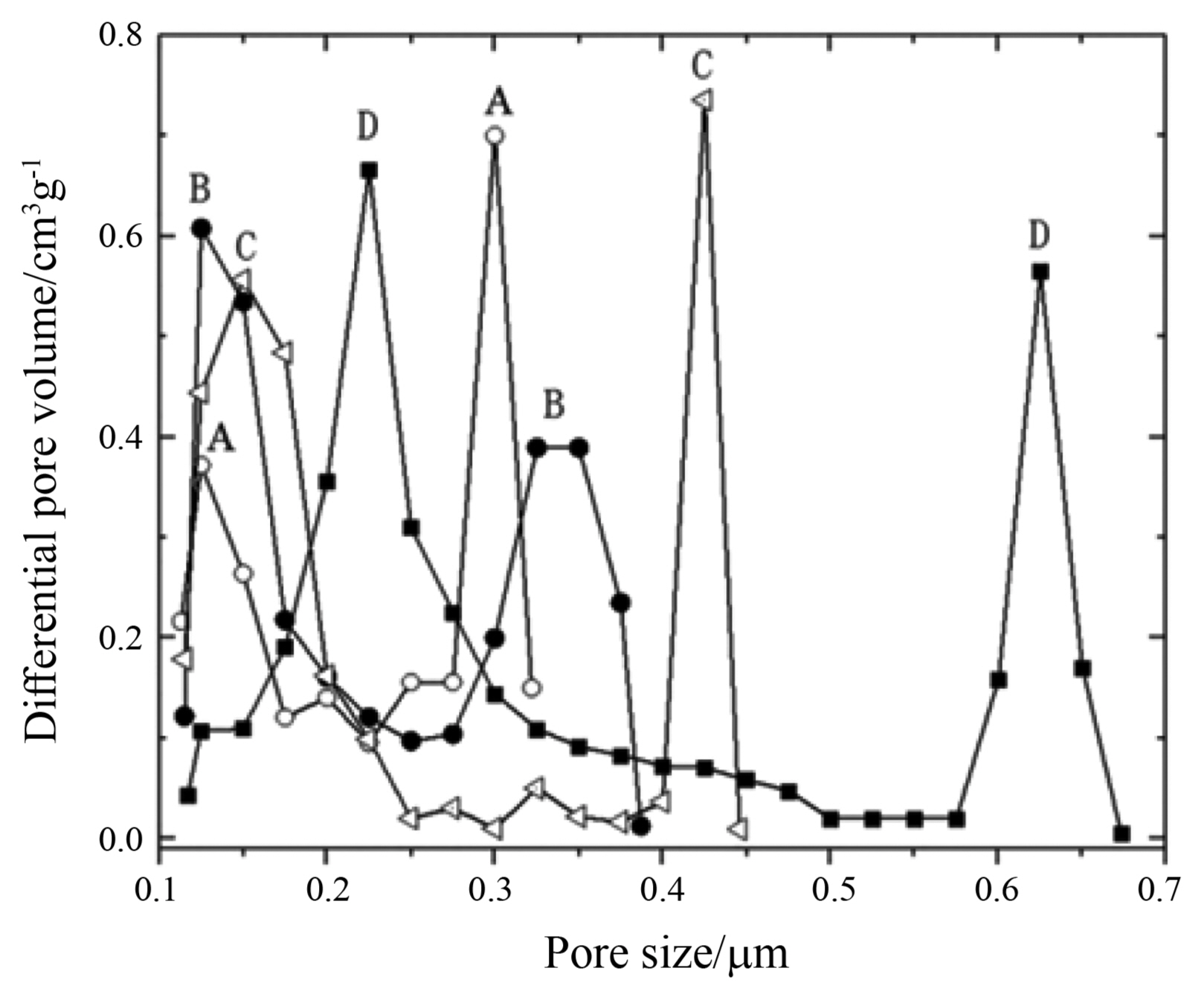
Figure 4 shows the pore size distribution of the porous Al2O3 ceramics sintered at 1350°C with various Al(H2PO4)3 additions. As revealed by Fig. 4, the pore size distribution of the porous Al2O3 ceramics with various Al(H2PO4)3 additions was bimodal. Furthermore, the pore sizes corresponding to the two peaks of the Al2O3 porous ceramics both increased appreciably with an increase in the amount of Al(H2PO4)3 addition. In other words, the Al(H2PO4)3 addition enlarged the average pore size of the porous Al2O3 ceramics, which could be mainly attributed to the evaporation of gaseous H2O and P2O5 resulting from the decomposition of Al(H2PO4)3 during sintering.
4. Conclusions
Porous Al2O3 ceramics were successfully fabricated with 0, 5, 10, and 20 wt% Al(H2PO4)3 additions. The open porosity and average pore size of the as-sintered porous ceramics increased remarkably with an increase in Al(H2PO4)3 addition. The flexural strength of the porous ceramics first increased to a peak value when 5 wt% Al(H2PO4)3 was added and then decreased as additional Al(H2PO4)3 was added. The average Al2O3 grain size of the porous ceramics changed in an opposite way as the flexural strength. It should be noted that the ceramics with 10 wt% Al(H2PO4)3 addition exhibited flexural strength comparable to that of the ceramics without Al(H2PO4)3 addition, although the latter had much higher porosity.