1. Introduction
Historically, a common method of direct evaporative cooling for buildings using water has been found in many parts of the Middle East including ancient Persia and in Egypt, in the form of the ‘Maziara jar’, which consists of window screens that were built with holes or niches for water jars. The airflow around the porous jars, which were prepared with local clay, evaporates the water and depresses its temperature.
The cooling effect achieved by the evaporation of absorbed water from porous ceramic materials can be used to cool buildings, as well as pavement bricks for local heat islands during summer season. These materials that lead to evaporative cooling have been developed using porous ceramics made from several materials such as alumina, low grade silica, cordierite, glass and some silicate wastes.1-15)
In developing countries of Africa, the Middle East, India and Southeast Asia, a very simple, cheap and ecofriendly cooling system using cheap local raw materials and without using electrical energy for cooling is required.16) In our previous study, we found that cheap clay for pottery production was also a useful raw material for making porous ceramic plates for self-cooling by evaporation of absorbed water.17)
In this study, porous plates were prepared from Onggi clay and bamboo charcoal powder by firing the two materials at 1100 and 1200°C, and the role of the porous properties in self-cooling of these porous plates via evaporation of absorbed water was investigated. A simple and new cooling system using porous plates and water was proposed.
2. Experimental Procedure
2.1. Preparation of porous ceramic plate from clay and bamboo charcoal powder
Onggi clay for old Korean pottery was used for making porous plates, and two kinds of commercial bamboo charcoal powder with different particle sizes were separately mixed with clay to form pores in the plates after firing. The average particle sizes of Type A and B charcoals (Type A and Type B) were determined to be 67 and 140 mm, respectively. The content of charcoal powder mixed with clay was 10 mass% and the water content in clay was 22.2 mass%. The charcoal powder was well mixed with clay and then put in a gypsum mold (65 × 11 × 8, and 11 × 11 × 1 cm) and pressed using 0.15 MPa pressure to prepare clay and charcoal composites. After drying plates at 75°C for 12 h, samples were fired at 1100 and 1200°C for 1h to burn off charcoal powder under air atmosphere. The heating rate, up to 1100 and 1200°C, was 150°C/h. Porous properties were analyzed using an Hg-Porosimeter (Model: Auto Pore IV, Micrometrics Instrument Co., USA) and the specific surface area was measured by BET method with nitrogen-gas as the adsorbent (Model: TriStar II, Micromeritics Instrument Co., USA). Minerals present in raw clay and fired plates were analyzed by X-ray diffraction (XRD) (Model: D/Max, 250, Rigaku Co., Japan). Chemical composition of clay was analyzed by X-ray fluorescence analysis (XRF)(Model: S1, TITAN, Bruker Co., USA). Microstructures of porous plates were observed using a field emission scanning electron microscope (FE-SEM) (Model: JSM-6390, JEOL Co., Japan).
2.2. Water absorption and air cooling effect of porous ceramic plate
Water absorption ability of porous plates fired at 1100°C was measured according to the moving rate of water from the bottom to top of porous plates at 27°C with 68% of relative humidity (R.H.). The porous plates were dried at 75°C for 24 h before water absorption test.
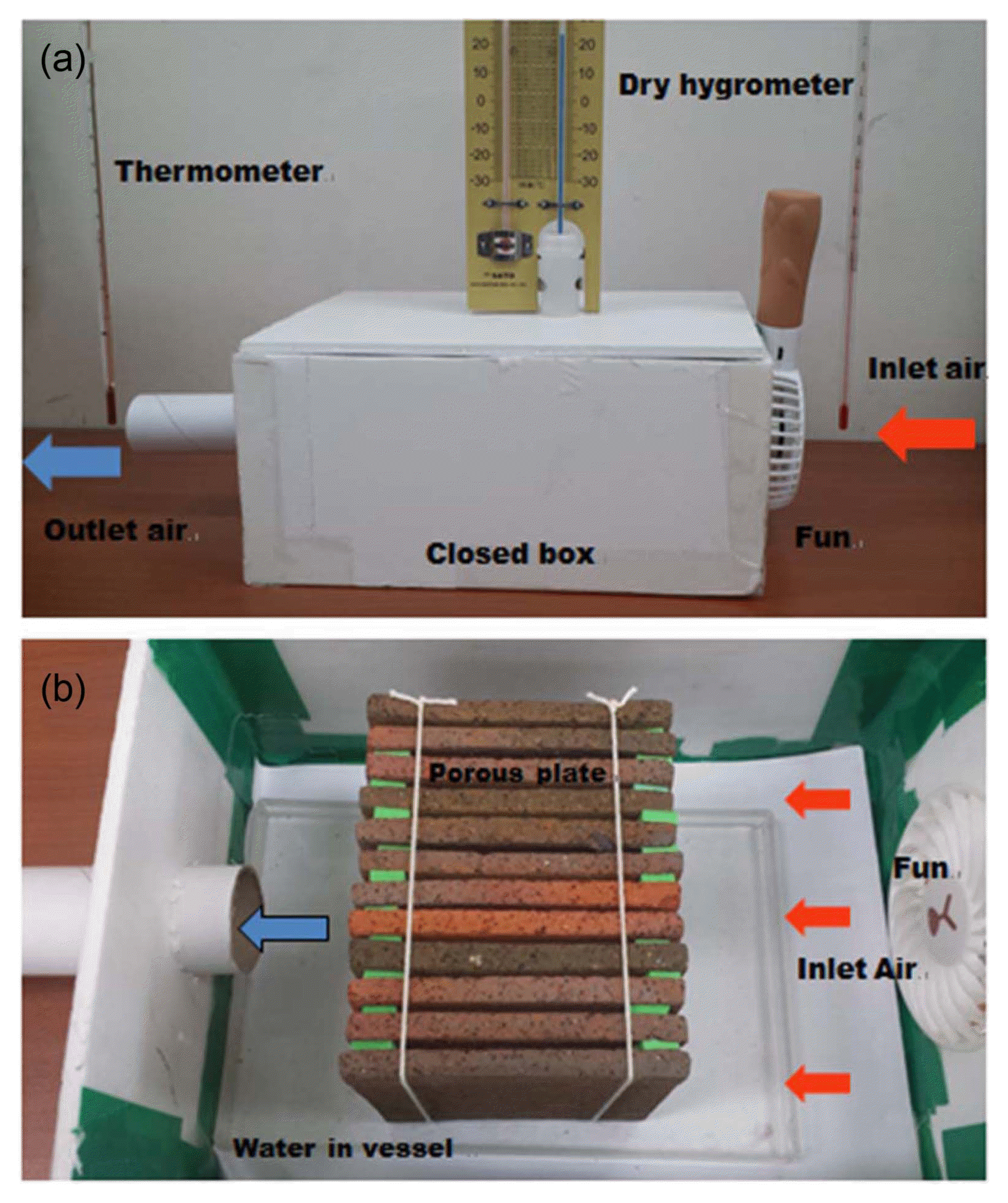
To investigate the cooling effect of porous plates via evaporation of absorbed water, 12 porous plates (9.5 × 9.5 × 0.6 cm) fired at 1100°C were set parallel to air flow in an adiabatic closed box (5400 cm3, 25 × 18 × 12 cm) assembled with polystyrene foam plates, as shown in Fig. 1, and approximately 800 cc/min of air was continuously introduced into the closed box by a small fan. This experiment was carried out in a temperature- and humidity-controlled room (ca. 60 m3). The inlet air was introduced from an 8 cm diameter window into the closed box (5,400 cm3) by a small fan; the cooled air was discharged via a 4 cm diameter pipe (Fig. 1). The linear velocities of the inlet and outlet air were 15.9 and 63.7 cm/min, respectively. In Fig. 1(b), the introduced air can be seen to pass through a space (250 cm3) surrounded by 12 porous plates (distance of two porous plates: 0.3 cm).
Temperatures at the inlet and outlet of the box were measured using a conventional glass thermometer. In this experiment, 200 cm3 of water was put into a plastic vessel (20 × 13 × 3 cm), which was set in the box; 1 cm of the lower part of the porous plate was dipped in water. Self-cooling tests were carried out at 25 - 30°C with 60 - 77% of R.H.
3. Results and Discussion
3.1. Porous properties of fired Onggi clay plates
The chemical composition of raw clay was determined to be as follows: SiO2 (56.05%), Al2O3 (24.8%), Fe2O3 (4.29%), TiO2 (0.57%), CaO (0.39%), MgO (2.67%), Na2O (tr.), K2O (1.69%) and loss of ignition (8.0 mass%). This clay had a small amount of coarse a-quartz particles of 0.5 to 2 mm of diameter after elutriation treatment of clay in water.
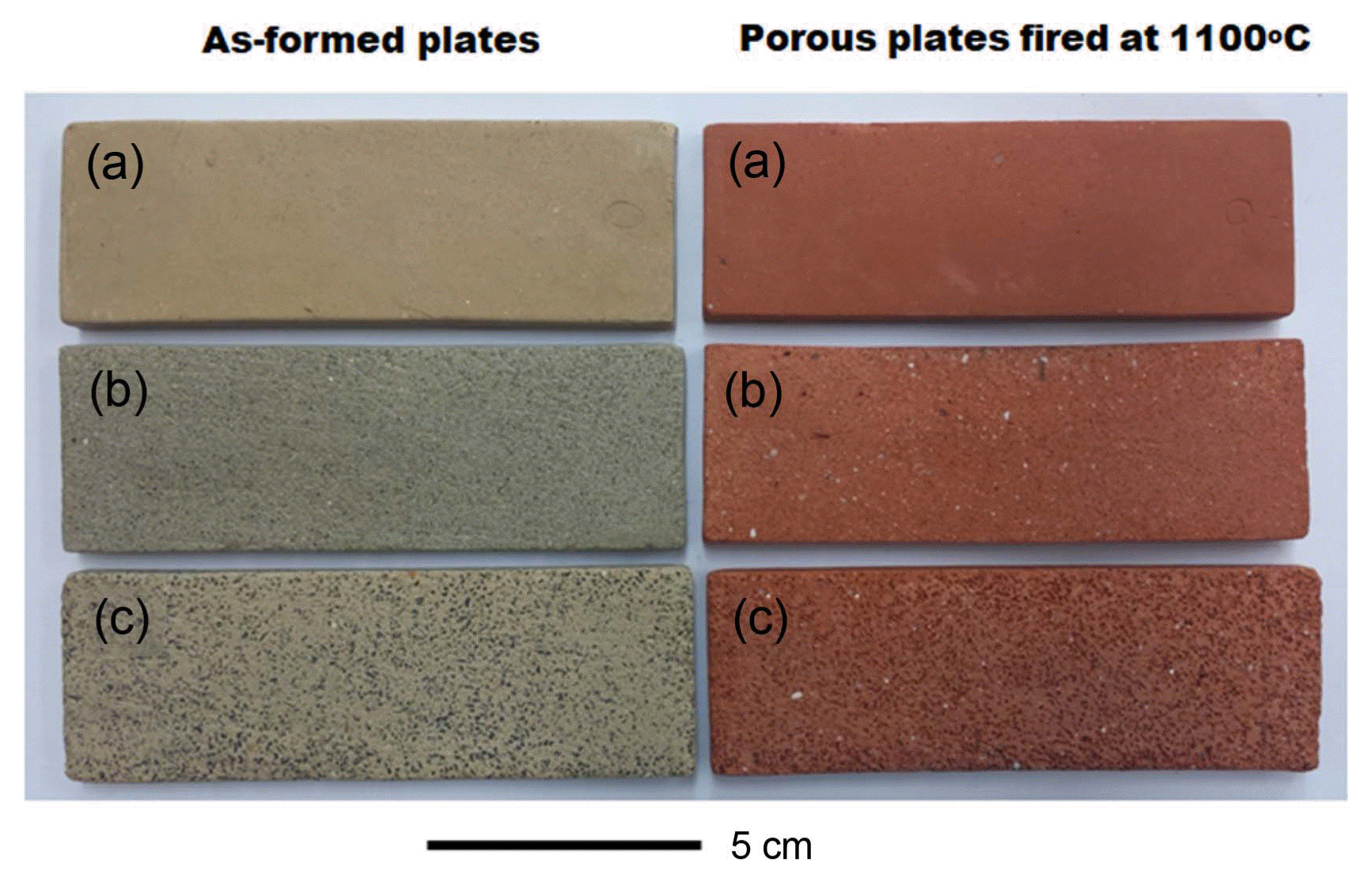
Clay plates with Type A and B powder were fired at 1100 and 1200°C for 1 h. Fig. 2 shows as-formed plates with two kinds of charcoal powder and the fired porous plates at 1100°C. Fig. 3 shows microstructures of the plates fired at 1100 and 1200°C. The sintering of the clay fired at 1200°C without charcoal powder (Fig. 3(b)) was promoted and formed glassy and round pores in the body due to bloating phenomenon.18)
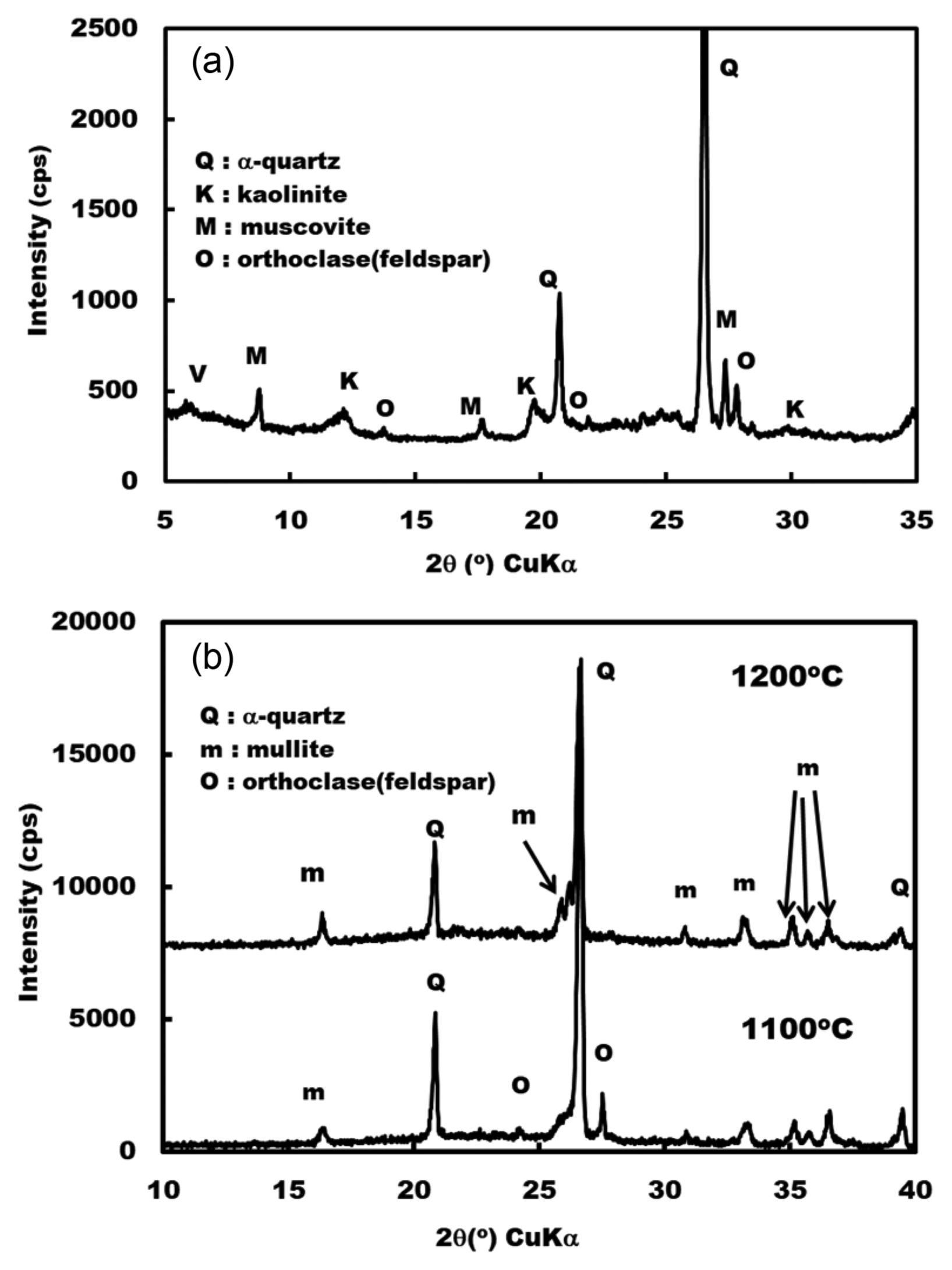
Figure 4 shows XRD patterns of raw clay and clays fired at 1100 and 1200°C. Raw clay was composed of a-quartz, kaolinite, K-feldspar and vermiculite. After firing at 1100°C, mullite phase, which was formed by the thermal decomposition of kaolinite and residual feldspar phase, was confirmed. At 1200°C, the plate was composed of a-quartz and mullite.
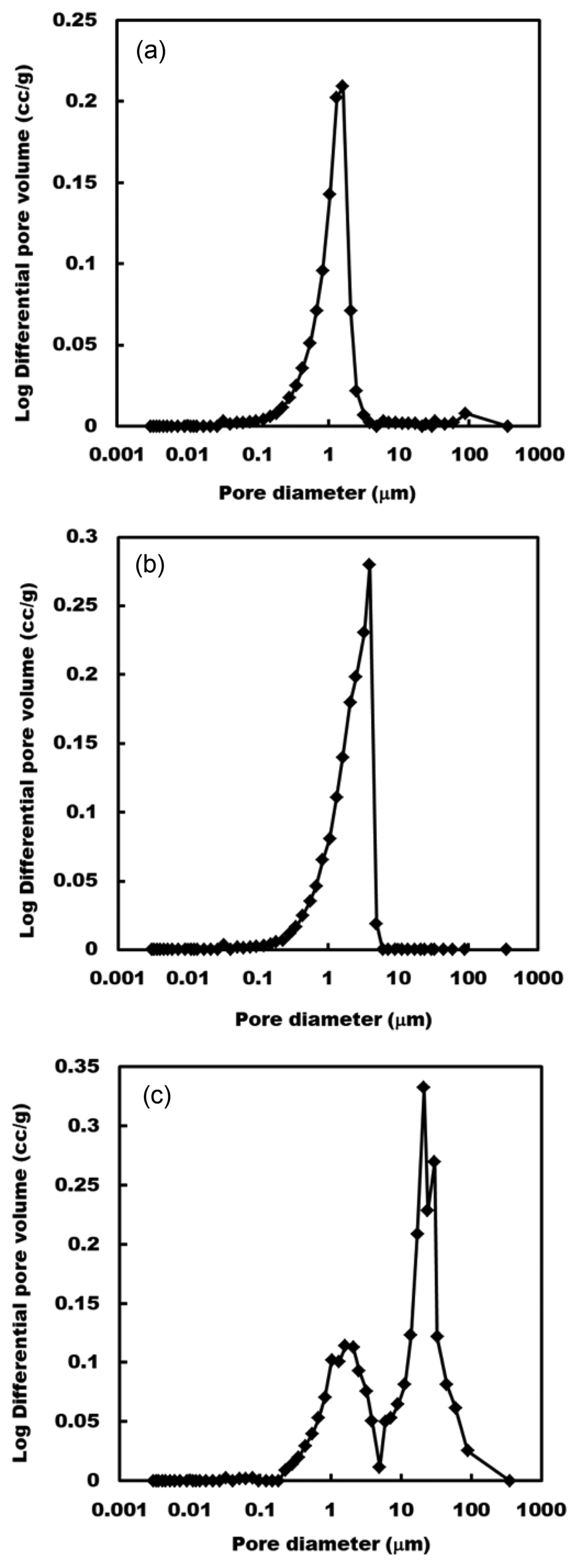
Figure 5 shows the pore-size distribution and porous properties of some porous ceramic plates fired at 1100°C in air with and without Type A and B powders. Plates fired without charcoal powder had porous structures with 20.9% of porosity and macropores; there was also a strong peak centered at around 1 mm. With the addition of Type A and B powders, porous plates had a duplex pore structure. Table 1 shows some properties of porous plates fired at 1100 and 1200°C. With the addition of two kinds of charcoal powder, the pore structure was controlled after firing at 1100 and 1200°C, as expected. Total pore volume and porosity, and bulk density of porous plates fired with two kinds of charcoal powder at 1100 and 1200°C, decreased with firing temperature. The clay plates fired at 1100 and 1200°C without charcoal powder had porous structures with 0.11 and 0.05 cm3/g of total volume, 0.81 and 0.33 mm of average pore diameter, and 20.9 and 10.8% of porosity, respectively. With the addition of 10 mass% of charcoal powder to the clay, porous plates with 1.27 and 2.56 mm of average pore diameter, 22.7 and 38.2% of porosity and 0.14 and 0.24 cm3/g of total volume were prepared from clay and Type A and B powders at 1100°C, respectively. Furthermore, specific surface area values of porous plates fired at 1100°C from clay and clay with Type A and Type B powders were 0.15, 0.45, and 0.54 m2/g, respectively. Porous plates with 1.17 and 2.03 mm of average pore diameter, 20.1 and 30.9% of porosity and 0.14 and 0.18 cm3/g of total volume were fired from clay and Type A and Type B powders at 1200°C, respectively (Table 1). In this study, porous properties of plates fired with charcoal powder were mainly influenced by particle size of charcoal powder firing temperature, but final average pore sizes of porous plate fired using Type A and B powders at 1100 and 1200°C did not depend on the 67 and 140 mm average particle sizes of the original Type A and B powders. The main reason for this lack of dependence of the porous properties on the particle size of the bamboo charcoal powder was probably the breakdown of fragile charcoal particles into smaller particles during mixing and kneading with clay, and pressing in the gypsum mold. This phenomenon was confirmed in the case of wood charcoal powder.17)
3.2. Water absorption porous ceramic plates
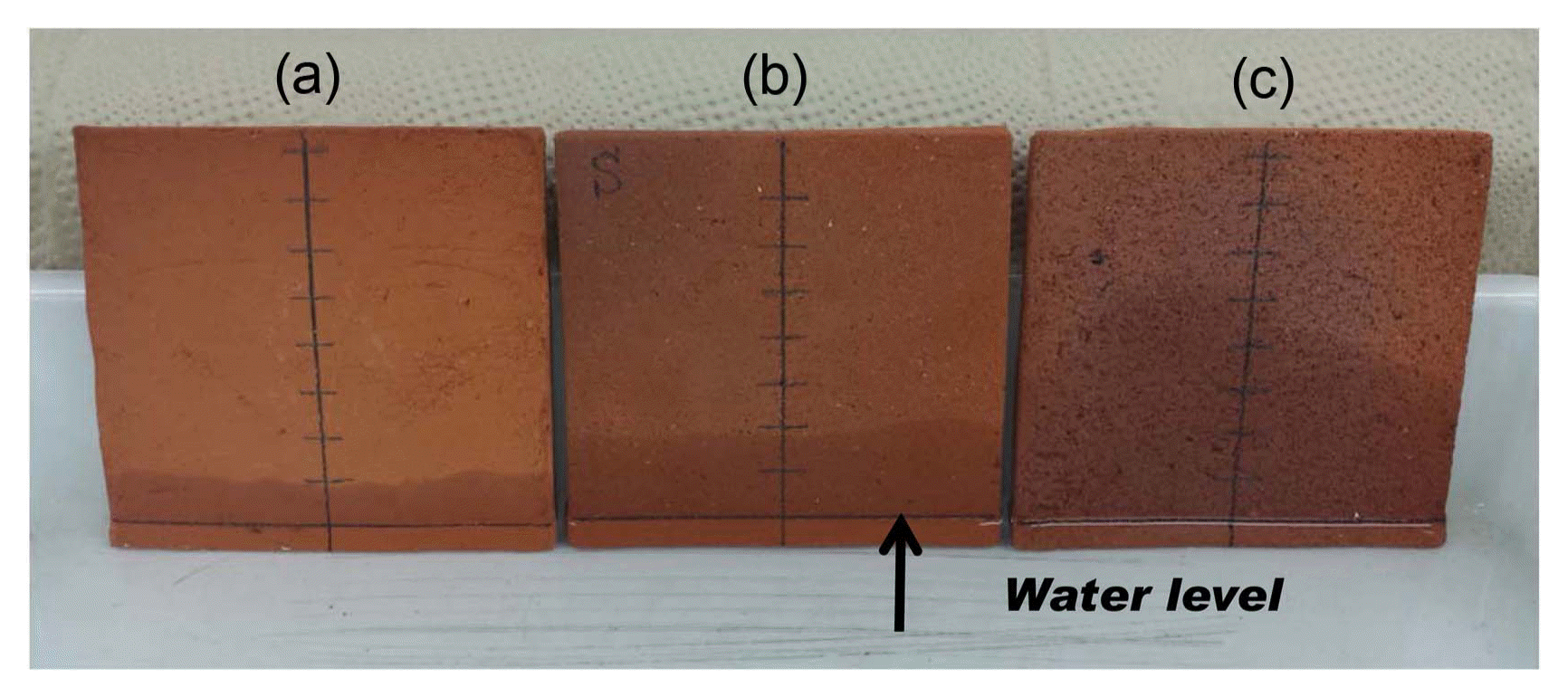
Figure 6 shows the method used to test the water absorption of porous plates fired at 1100°C. Pictures were taken after water was poured for 8 minutes at 27°C and 68% of R.H. It took about 40 min (Type B powder), 100 min (Type A powder) and 180 min (without charcoal powder) for the top of porous plates to be saturated with absorbed water. Average moving rates of water from the bottom to the top of the porous plates fired without charcoal powder were 0.53 mm/min and 0.95, and 2.4 mm/min for plates fired with Type A and B powders, respectively. Average moving rate of porous plates of Type B powder was 2 - 5 times faster than that of plates fired without charcoal powder. The amount of water absorbed in one porous plate after water reached the top of the plate prepared without charcoal powder was 13.1 g; for plates prepared with Type A and B powders, the values were 13.5 and 15.2 g.
3.3. Air cooling effect of porous plate via evaporation of absorbed water
Air cooling tests on porous plate surface were performed in an adiabatic closed box. The effect of the porous plate structure on the air cooling effect was investigated. The porous plates were set parallel to the flow of introduced air (Fig. 1). Table 2 shows the effect of porous plates fired at 1100°C and R.H on air-cooling by water evaporation. Number of porous plates was 12 and distance between two plates was 3 mm. The inlet air was introduced after the top of each plate was immersed in the absorbed water. Inlet air samples at temperatures and relative humidity levels of 26°C and 60%, 28°C and 77% and 30°C and 72% were introduced into the box; the temperature of the cooled air was measured at the outlet of the box. The temperatures at the inlet and outlet were measured every 10 min after introducing air; the average temperatures (T1 and T2) were calculated and are shown in Table 2. By passing air through porous plates, cooled air at the outlet was obtained. Cooling effect values (ΔT) were 0.8 - 1.3°C at 28°C (at 77% of R.H.), 1.4-2.2°C at 30°C (at 72% of R.H.) and 2.3 - 3.6°C at 26°C (at 60% of R.H.). The cooling effect was dependent on R.H. and the porous properties of the added charcoal powder. It was possible to obtain cooled air (ΔT = 3.5 - 3.6°C) using porous plates fired from clay with Type A and B charcoal powders at 60% of R.H. and 26°C (Table 2). Porous plates fired from clay without charcoal powder showed a cooling effect lower than that of porous plates prepared from clay with Type A and B charcoal powders. Porous plates fired from clay with Type B powder at 1100°C had higher total pore volume, average pore diameter, and porosity than those characteristics of porous plates fired from clay with Type A powder (Table 1). The average evaporation rates of absorbed water from the surface of one porous plate over 3 h at 25°C with 60% of R.H. was 7.9 g/min for the porous plate fired from clay without charcoal powder, 13.3 g/min for the plate fired from clay with Type A powder, and 14.1 g/min for the plate fired from clay with Type B powder. From these results, it can be seen that the evaporation rate of water from the surface of porous plates has an important role in generating cooled air by evaporation of absorbed water.
Table 3 shows the effects of stacking of porous plates and of the distance between two porous plates fired at 1100°C from clay with Type B powder on air cooling by water evaporation. With increasing humidity in the inlet air, the self-cooling effect (ΔT) decreased due to suppression of water evaporation from the surface of the porous plates. 12 stacked porous plates with narrow distance (< 1 mm) between them showed a lower self-cooling effect (ΔT = 1.5°C at 27°C with 70% of R.H. and ΔT = 1.8°C at 25°C with 64% of R.H.). With increasing of the distance between stacked plates, contact of inlet air with the surface of the plate was promoted, enhancing the cooling effect of water evaporation. Furthermore, the self-cooling effect was promoted with increasing of the number of the stacked porous plates, as expected.
In this study, the effects of the properties of porous ceramic plates, and of the number and distance between plates fired at 1100°C from Onggi clay and charcoal powder, on self-cooling of air was investigated. Study is in progress of the effects of the thickness of porous plate, surface roughness of plate and contact method of inlet air and porous plates on the high self-cooling effect of this simple and ecofriendly system.
4. Conclusions
In this paper, some porous ceramic plates formed using Onggi clay and bamboo charcoal powder were prepared at 1100 and 1200°C, and certain porous properties, including the water absorption and the cooling effect, according to the evaporation of absorbed water in a closed box, were investigated to produce an eco-friendly and simple cooling system. Porous properties were dependent on the firing temperature and the addition of wood charcoal powder. Total pore volume, average pore size and porosity were 0.11 - 0.243 cm3/g, 0.81 - 2.56 μm and 20.9 - 38.2%, respectively at 1100°C, and 0.049 - 0.182 cm3/g, 0.33 - 2.03 μm and 10.8 - 30.9%, respectively at 1200°C. Self-cooling effect of porous ceramic plate with absorbed water was investigated under flowing air in a closed box; effect was found to be dependent of porous properties and relative humidity of inlet air. Porous ceramic plates with 22.7 - 38.2% of porosity and 1.27 - 2.56 μm of pore diameter were found to be able to produce cooled air with 3.5 - 3.6°C of temperature drop at 26°C and 60% of relative humidity. The self-cooling effect was promoted with increasing of the number of stacked porous plates and distance between plates.