1. Introduction
The synthesis of TiO2 nanotubes using TiO2 nanoparticles was first reported in the microstructure analysis results of T. Kasuga in 19981) for a synthesized powder obtained from the reaction between the 2.5 - 20 M NaOH aqueous solution and TiO2-SiO2 powder, synthesized using the solgel method. Afterwards, the TiO2 nanotube synthesis mechanism from the NaOH aqueous solution reaction was reported in the following study in 1999.2) In the NaOH aqueous solution reaction, Na+-O-Ti bonds are formed in place of the broken Ti-O-Ti bonds on the distilled water processed TiO2 surface and electrostatic equilibrium is maintained. On the other hand, the charges that did not form bonds and remain on the TiO2 surface cause the charged crystals to separate and minimize electrostatic repulsion. However, when reacted with the concentrated HCl aqueous solution, the electrostatic repulsion dissipates, and the crystals adhere to each other and form particles.
In contrast, when reacted with distilled water, the Ti-O-Na bond becomes Ti-OH bonds and these bonds are also formed on the crystal surface by the residual charge, resulting in sheet forms containing the tube structures. Also, when reacted with HCl aqueous solution, Ti-O-Ti bonds or Ti-O···H-O-Ti hydrogen bonds are formed through the dehydration reaction of Ti-OH. As a result, the distances between Ti on surface are decreased and result in the folding of the sheets. In this process, the ends of the sheets become connected to form tube structures due to the electrostatic repulsion from the Ti-O-Na bonds on the TiO2 crystal surface.
The advantages and disadvantages of such synthesized TiO2 nanotubes have been reported and include the following. 3-8) 1) Mass production is possible due to the low investment costs for the synthesis equipment; 2) enhancement of photocatalytic properties is possible through microstructure control including metallic or nonmetallic doping, chemical compound adhesion, and surface nanostructuring; and 3) the cation exchange capacity is high. Disadvantages include 1) the long reaction time and the need for high concentrations of NaOH of about ~ 10 M for the synthesis and 2) it is difficult to obtain nanotubes of uniform size.
After the TiO2 nanotube synthesis was reported, J. Suetake studied the influence of microstructure and photocatalyst property variation on Na ions in 2008. Suetake analyzed the 23Na and 1H nuclear magnetic resonance (NMR) and found that the Na ions of the NaOH aqueous solution used in the TiO2 nanotube synthesis strongly bond between the nanotube sheets to stabilize the tube structure, or form weak bonds to the nanotube surface and reduce the photocatalytic properties. While this problem can be resolved by removing the weakly bonded Na ions using a 50 mM HNO3 aqueous solution, control of the Na ion content is important to maintain the nanotube structure.
Additionally, from trimethylamine, acetaldehyde decomposition testing of the TiO2 nanotubes synthesized with TiO2 nanoparticles (P25), it was reported that the amount adhering to the nanotube surface was 3 times that of nanoparticles, and the HNO3 processed nanotubes removed the Na ions on the surface to reduce the recombination rate of the electrons and holes to enhance photocatalytic properties. 9) While basic research has been conducted on the mechanism and synthesis of TiO2 nanotubes using TiO2 nanoparticles, studies comparing the photocatalytic properties of the TiO2 nanoparticles and TiO2 nanotubes using the TiO2 nanoparticles are less reported.
In this study, transmission electron microscopy (TEM) analysis was used to observe the TiO2 in nanotube form where the atomic layers of the single crystal TiO2 nanoparticles of 15 nm average diameter were divided into 2 walls (0.9 nm) and 3 walls (1.4 nm). Also, the photocatalytic reduction reaction test of oxidized methylene blue revealed that the reduction reaction rates by the nanotubes and nanoparticles were 0.24 μmol/s and 0.09 μmol/s, respectively, and the reaction rate property was enhanced by 2.6 times compared to that using nanoparticles.
2. Experimental Procedure
For the synthesis of TiO2 nanotubes, 8 g of NaOH (Extra Pure, OCI Company Ltd) was dissolved in 20 mL of distilled water (18.3 MΩ·cm) and 0.2 g of TiO2 nanoparticles (P25 AEROXIDE, CAS-REG.NR. 13463-67-7) were added to the prepared solution. (Since the temperature of the solution increases rapidly when NaOH is added to distilled water, it was dissolved in 1 g intervals over 8 times and cooled to room temperature before the TiO2 nanotubes were added. This was done to exclude the effects of the TiO2 nanotube microstructure change due to the increased temperature during the dissolution.) The solution with the dispersed TiO2 nanoparticles was prepared in glass bottles and then placed in a convection oven at 110°C for synthesis over 24 h. After 24 h, the bottles were removed from the convection oven and cooled using tap water. Then the lids were opened to pour running distilled over into a 1 L glass beaker and a glass rod was used to collect the TiO2 powder that adhered to the bottom of the glass bottles.
For pH neutralization of the recovered powder, a 1 L glass beaker was filled with distilled water and the solution excluding the precipitated TiO2 powder was newly replaced. This was carried out 5 times and then a vacuum filtration device using cellulose paper filter (ADVANTEC, 5C) was used to collect the TiO2 powder followed by drying in the convection oven at 50°C for 12 h. The microstructure of the TiO2 nanoparticles and TiO2 nanotubes synthesized using the TiO2 nanoparticles were analyzed using a 300 keV in-situ transmission electron microscope (JEM 3011 HR) and X-ray diffractometer (SWXD_D-MAX/2500-PC, CuKα).
For the photocatalytic property comparison, 0.374 mg (10 μmol) of methylene blue (trihydrate, DUKSAN, Lab Grade) brown powder was dissolved in 100 mL of distilled water and 3.5 mL of the prepared blue color methylene blue solution was poured in a Quartz cuvette (1 mm × 1 mm × 40 mm, Starna scientific). A UV-Vis spectrometer (UV-2600, SHIMADZU/JAPAN, Detronium (D2) lamp + Tungsten (W) lamp) was used to measure the absorptivity in the wavelength range of 185 nm - 900 nm then a thermopile detector (818P-001-12, NEWPORT/USA) and power meter (1918-C, NEWPORT/USA) were used to control the intensity of the white light irradiated from the 1 kW Xe-arc lamp (IR filtering using a water filter) to 100 mW/cm2. Then, 5 mg of the TiO2 nanoparticle or nanotube powder was added to 3.5 mL of the oxidized methylene blue solution and the solution underwent ultrasonic treatment over 6 rounds in 10 s intervals (for uniform dispersion of the powder within the solution). Afterwards, the solution was exposed to white light irradiation for 60 s and a centrifuge (OHAUS, 6,000RPM) was used for 10 minutes at 6,000RPM to settle the TiO2 nanoparticles. Then, only the solution was moved to the Quartz cuvette and the absorptivity in the wavelength range of 185 nm - 900 nm was measured.
3. Results and Discussion
The microstructure changes in the TiO2 nanoparticles were clearly observed using TEM imaging. In low magnification images, polygonal TiO2 nanoparticles with an average diameter of 15 nm were observed as shown in Fig. 1(a) while Fig. 1(b) shows the nanoparticles observed in Fig. 1(a) at a higher magnification. The single crystal TiO2 nanoparticles in Fig. 1 were distinguishable by their anatase phase (#01-075-1537, JCPDS, Tetragonal, I41/amd(No.141)) with a spacing of 1.23 Å between the crystal planes.
Figure 1(c) shows the electron diffraction pattern measurement result in a region of low magnification images including numerous nanoparticles. Based on the transmitted beam, diffraction occurred at the (110), (111), and (211) crystal planes of the rutile phase (#01-070-7347, JCPDS, Tetragonal, P42/mnm (No.136)). Fig. 2(a) and (b) show the TiO2 nanotube synthesis with the folding of the Ti-OH sheets, which appeared in the nanotubes synthesized using the TiO2 nanoparticles. Fig. 2(c) shows the mixture of TiO2 nanotubes mixed together with Ti-OH sheets. Fig. 2(d) shows the magnified images of the changed TiO2 nanotube bundles.
From Fig. 2(e) and (f), the 2 wall (0.9 nm) and 3 wall (1.4 nm) atomic layers of the single crystal TiO2 nanoparticles were observed along with tubes with 3 or more walls and rolling sheet formations. Fig. 2(g) shows the electron diffraction pattern of the TiO2 nanotubes. Based on the transmitted beam, diffraction occurred at the (004) crystal plane of the anatase phase and the (210), (301) crystal planes of the rutile phase. The significant difference between the diffraction patterns of the TiO2 nanoparticles and nanotubes was that an amorphous electron diffraction pattern was obtained when they changed to nanotubes.
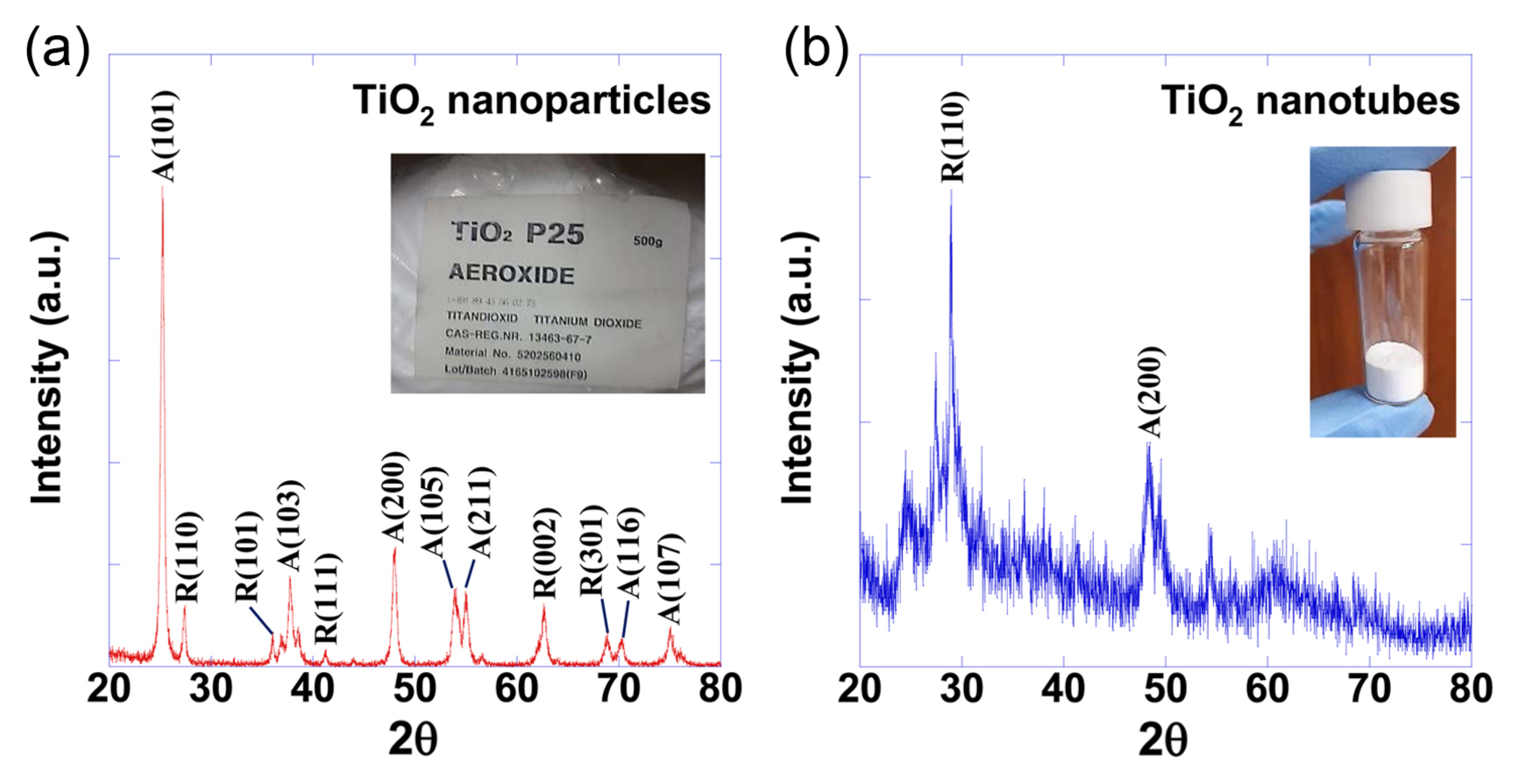
The amorphization observed when the TiO2 nanoparticles were changed to nanotubes was also observed in the X-ray diffraction patterns, which are shown in Fig. 3(a) for the TiO2 nanoparticles and in Fig. 3(b) for the TiO2 nanotubes. In the X-ray diffraction pattern for the TiO2 nanoparticles, there was a significant number of particles with the anatase (101) crystal plane, while the X-ray diffraction pattern for the nanotubes showed a significant number of particles with the rutile (110) crystal plane.
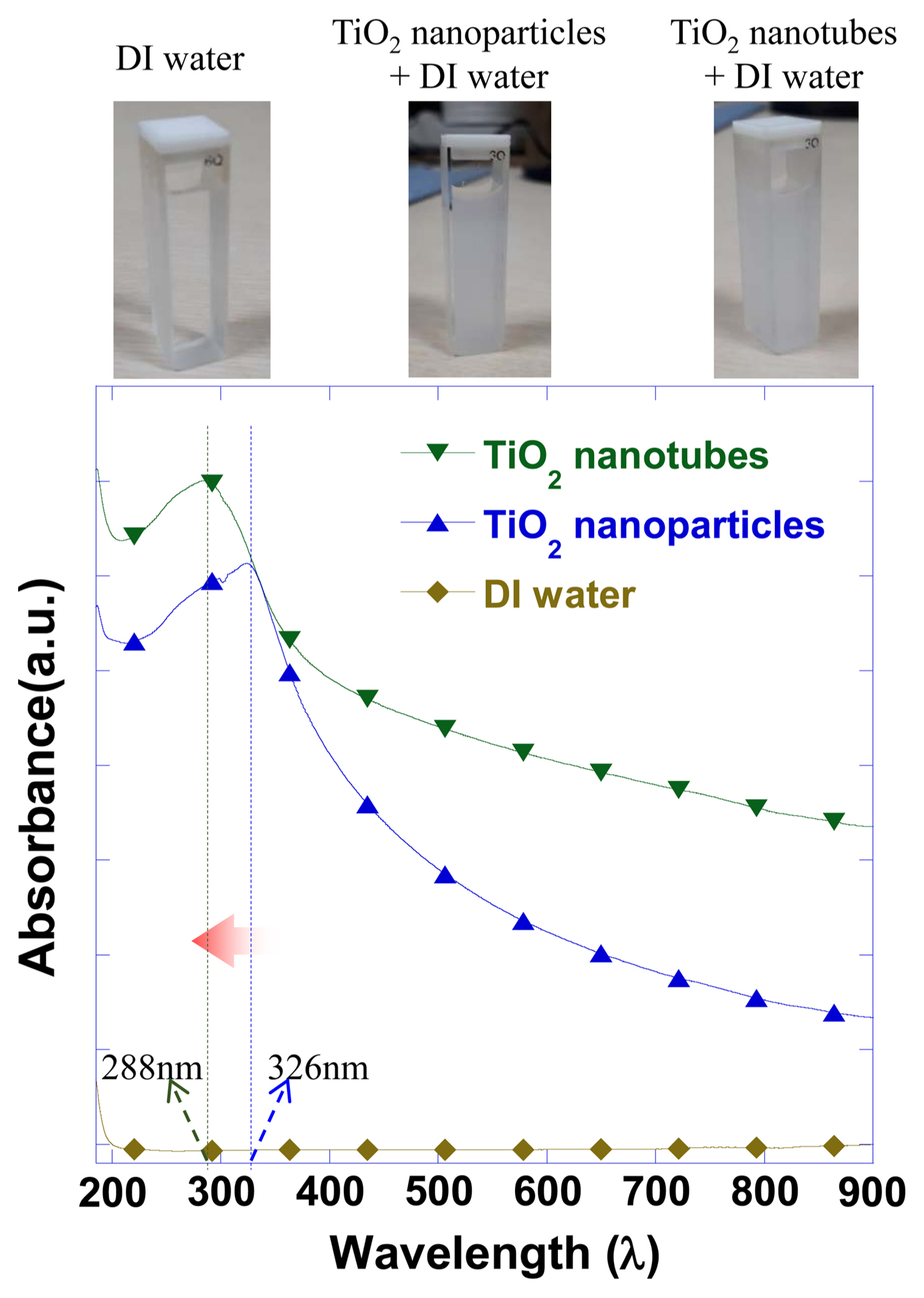
Before comparing the photocatalytic properties of the TiO2 nanoparticles and TiO2 nanotubes synthesized using TiO2 nanoparticles, the absorptivity of the TiO2 dispersed in distilled water was measured for the wavelength range of 185 - 900 nm. The measurement results shown in Fig. 4 revealed that the UV absorption peak shifted from 326 nm to 288 nm. This shift was thought to be due to the band gap increase produced by the crystal refinement and amorphization.
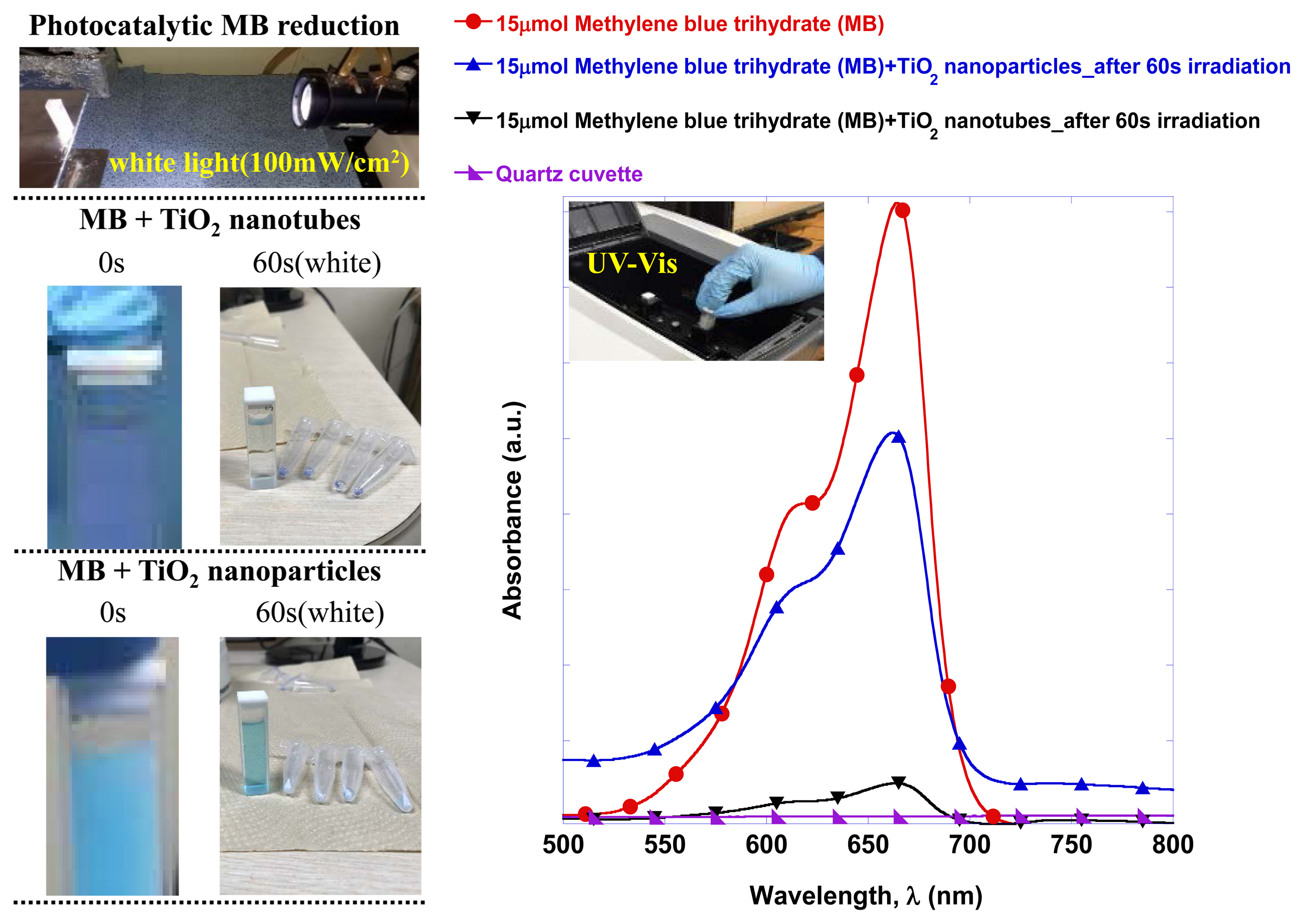
By comparing the photocatalytic reduction reaction of oxidized methylene blue using nanoparticles and nanotubes through the UV-Vis absorptivity measurement, clear differences were observed and the results are shown in Fig. 5. The first visible difference was that the dispersion of TiO2 nanoparticles in the same oxidized methylene blue solution gave off a light blue color, while dispersion of the TiO2 nanotubes resulted in a deep blue color. Irradiation of white light for 60 s followed by TiO2 powder separation using a centrifuge resulted in insignificant differences in the solution colors. Using TiO2 nanotubes resulted in a virtually transparent state visibly and the absorptivity measurement result showed that the absorptivity of the oxidized methylene blue was reduced by 94.7%.
In Fig. 6, the oxidized methylene blue reduction reaction rate is quantitatively compared according to the white light exposure time using the measured absorptivity comparison results. It was found that the reduction reaction rate of the TiO2 nanoparticles was 0.09 μmol/s and the reduction reaction rate of the TiO2 nanotubes was 0.24 μmol/s, or a 2.6 fold enhancement.
In this study, the process from single crystal TiO2 nanoparticles to nanotube structures was determined by TEM imaging to involve the separation of the atomic layers and the rolling phenomenon of the separated layers. It was predicted that in this process the crystallinity of the nanoparticles is destroyed, and the amorphous and crystalline are mixed, decreasing the photocatalytic efficiency. However, comparison of the photoreduction reaction rate of the oxidized methylene blue revealed that the nanotube characteristic was improved by 2.6 times. Based on these results, it was determined that the surface area and nanotube structural characteristics have a greater impact on the crystallinity of TiO2 photocatalysts over the photocatalytic properties. Furthermore, the difference in color when dispersing TiO2 nanotubes in the oxidized methylene blue solution was determined to be a result of the quantum confinement phenomenon, equivalent to the optical band gap increase phenomenon, like quantum dots (CdS, CdSe, etc.). Sizes smaller than the nanoparticles along with amorphization were observed when the TiO2 nanoparticles changed into nanotube structures.
4. Conclusions
In this study, the synthesis of TiO2 nanotubes using TiO2 nanoparticles and their photocatalytic properties were investigated. 10 M NaOH was used to synthesize TiO2 nanotubes using TiO2 nanoparticles, and TEM imaging was carried out to observe the nanotubes resulting from the rolling phenomenon of the separated single crystal TiO2 atomic layers.
The photocatalytic properties were analyzed using UV-Vis absorptivity measurements of the oxidized methylene blue reduction process. The concentration function of the oxidized methylene blue according to time revealed that the reduction reaction rate of the single crystal TiO2 nanoparticles was 0.09 μmol/s and the reduction reaction rate of the synthesized nanotubes was 0.24 μmol/s. The synthesized nanotube structures photocatalytically oxidized methylene blue 2.6 times better. From the XRD analysis results, the crystallinity of the nanotubes was determined to have decreased compared to that of the nanoparticles, however, the increased photocatalytic property is thought to be due to the larger surface area and structural characteristics of the nanotubes.
Such methods of controlling physicochemical characteristics using various microstructural changes of photocatalytic materials is expected to contribute to the enhancement of CO2 conversion, fuel and hydrogen generation from solar water splitting utilizing conventional photocatalytic methods, and the efficient decomposition of environmentally hazardous substances, including volatile organic compounds.