1. Introduction
Zinc oxide (ZnO) and titanium dioxide (TiO2) are n-type semiconductor oxides that have attracted a lot of research interest. Both ZnO and TiO2 possess outstanding physical and chemical properties such as non-toxicity and high reactivity at the nanoscale. Furthermore, both are highly earth-abundant, and hence cheap. ZnO and TiO2 also exhibit wide bandgaps of 3.37 and 3.2 eV (anatase) respectively.1-3)
TiO2 in the anatase phase shows excellent photocatalytic activity and is widely used for photocatalytic processes like water-treatment and air-purification. This is because it has a high oxidizing potential for the photo degradation of organic and inorganic compounds and dyes. ZnO also exhibits high photocatalytic activity and can effectively decompose certain compounds even in the dark. Unfortunately, the photocatalytic efficiencies of both TiO2 and ZnO are limited by certain factors. Their wide bandgaps permit UV light absorption only at wavelengths lower than or equal to 385 nm (λ ≤ 385 nm). In addition, the high recombination rate of the electron-hole pairs generated by these oxides also degrades their photocatalytic efficiency.3,4)
To overcome this limitation, various efforts have been made to develop nanocomposites containing both these oxides. Such nanocomposites can absorb visible light with wavelengths ≥ 400 nm and also offer enhanced charge separation.4,5) There have been reports on the wet chemical synthesis of ZnO/TiO2 core-shell nanostructures.6) These core-shell structures were found to be more effective for the photochemical degradation of acridineorange (in the presence of sunlight) than TiO2 and ZnO nanostructures. These core-shell structures also exhibited a wide absorption spectrum extending up to the visible-light region. Kanjwal et al.7) synthesized a ZnO/TiO2 nanocomposite using electrospinning and a hydrothermal method. The resulting ZnO/TiO2 nanocomposite showed better photocatalytic degradation of dye pollutants than pure ZnO and TiO2. These results suggest that the optical and photochemical properties of pure TiO2 and ZnO can be improved by developing nanostructures consisting of both of these oxides. In this study, we synthesized a ZnO/TiO2 nanocomposite with excellent photocatalytic properties via a reproducible, cost-effective, and versatile modified sol-gel technique.8)
The ZnO/TiO2 nanoparticles were synthesized by a sol-gel method using zinc acetate dihydrate. The titania content in the nanoparticles was varied and its effect on the morphology, crystal structure, and optical properties of the resulting nanoparticles was investigated.
2. Experimental Procedure
2.1. Materials
Zinc acetate dihydrate (≥ 98%, Sigma-Aldrich) was used as the ZnO precursor without further purification. Sodium hydroxide was used as the precipitating agent and de-ionized water as the solvent for all the experiments. Titanium oxysulfate (29%, Sigma-Aldrich) was employed as the titania precursor while ammonium hydroxide (28%, Sigma-Aldrich) was used as the pH adjuster.
2.2. Synthesis of ZnO/TiO2 nanocomposite
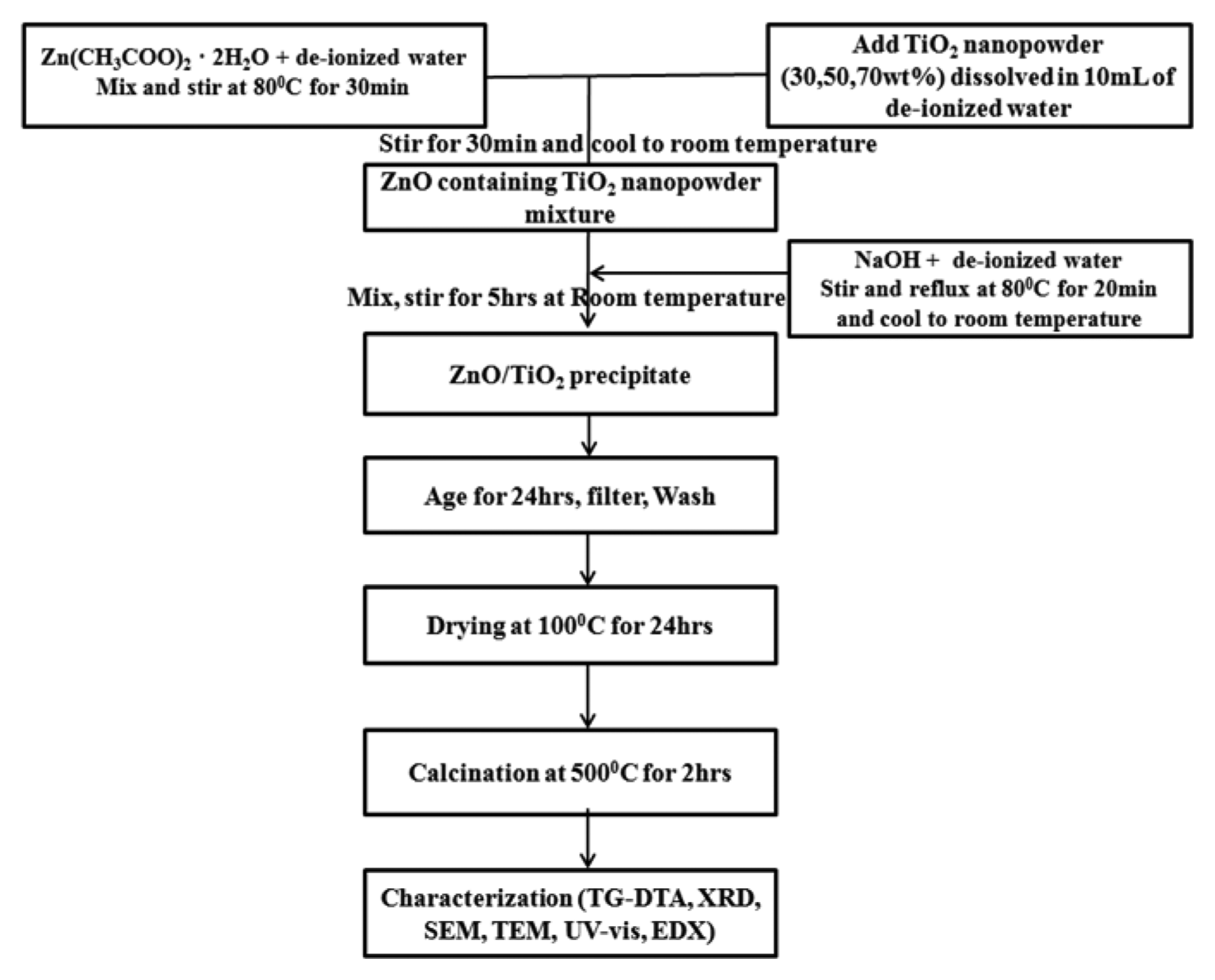
The ZnO/TiO2 nanocomposites were synthesized by incorporating TiO2 nanopowder into the ZnO sol-matrix. This TiO2 nanopowder was synthesized by modifying the method proposed by Nankya et al.9) The resulting TiO2 nanopowder was dried at 100°C for 3 h. A weighed sample of zinc acetate dihydrate was dissolved in de-ionized water to form a 0.2 M solution, which was magnetically stirred at 80°C for 30 min. The TiO2 nanopowder was then added to the ZnO solution. The resulting mixture was stirred for another 30 min before being cooled to room temperature. An aqueous solution of sodium hydroxide was prepared and stirred at 80°C for 20 min. This solution was then cooled to room temperature. The cooled sodium hydroxide solution was added drop-wise to the ZnO/TiO2 solution at room temperature and the resulting solution was stirred vigorously for 5 h. The ZnO/TiO2 precipitate so obtained was aged for 24 h, after which it was filtered and washed several times. It was then dried at 100°C for 24 h, calcined at 500°C for 2 h, and ground into powder. Fig. 1 shows the schematic of the procedure used for the synthesis of the ZnO/TiO2 nanocomposites.
2.3. Characterization
X-ray diffraction (D/Max-2200, Rigaku, Japan) was carried out to examine the crystal structure and calculate the crystallite size of the nanocomposites. Field emission scanning electron microscopy (FE-SEM, JEOL, JSM-6701F, Japan) and energy dispersive X-ray spectroscopy (EDX, line-scan mapping) analyses were carried out to examine the microstructure and elemental composition of the nanocomposites. The morphology of the ZnO/TiO2 nanocomposites was examined using transmission electron microscopy (TEM, JEOL, JEM-2100F, Japan). UV-vis spectroscopy (Shimadzu, UV-2600) was carried out to investigate the optical properties of the nanocomposites.
3. Results and Discussion
3.1. Crystal structure
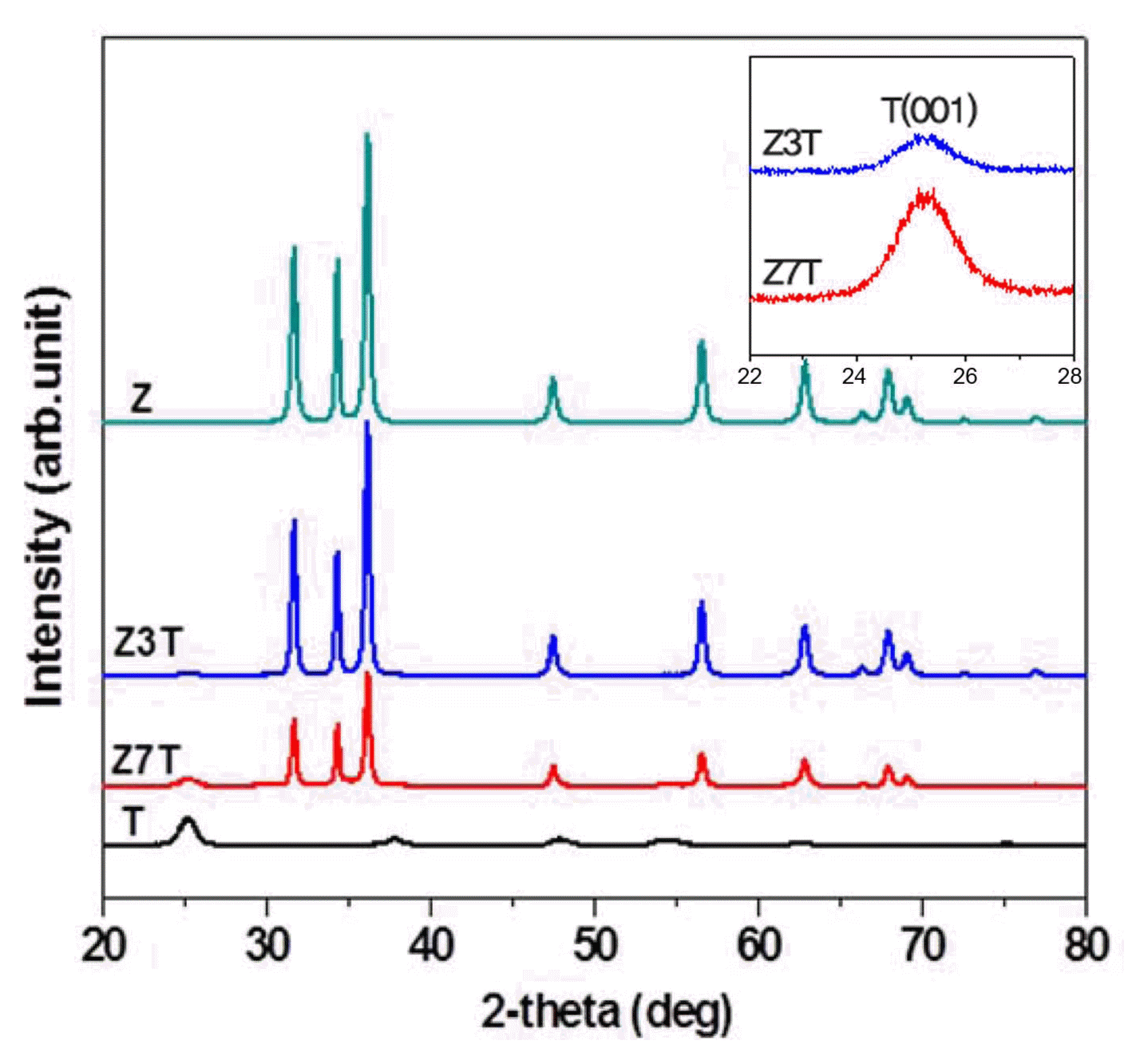
The XRD patterns of the calcined samples pure ZnO (Z) and TiO2 (T) and the ZnO/TiO2 nanocomposites (Z3T, Z7T)) are shown in Fig. 2. The peaks observed at 2θ = 31.69, 34.36, 36.17, 47.48, 56.52, 62.79, 66.34, 67.88, 69.04, 72.51, and 76.89 correspond to the ZnO hexagonal wurtzite phase (JCPDS card No. 36-1451). On the other hand, the peaks observed at 2θ = 25.24, 37.82, 47.91, 53.92, 62.78, 68.87, and 75.17 correspond to the TiO2 anatase phase (JCPDS card No. 21-1272). Z3T showed the (101) and (004) diffraction planes, while Z7T showed the (101), (004), and (105) diffraction planes. This indicates that the anatase phase was present in the synthesized nanocomposites. No impurity peaks were observed, indicating that the samples were pure.
The intensity of the XRD peaks decreased with an increase in the weight percentage of the TiO2 nanopowder added to the ZnO sol-matrix. The Z3T sample with 30 wt% TiO2 nanopowder showed intense XRD peaks. An increase in the TiO2 content increased the sharpness and intensity of the anatase phase peaks, as shown in the inset of Fig. 2.
3.2. Crystallite size
The crystallite sizes of the nanocomposites were calculated using the Scherrer equation:
where t is the crystallite size (nm), k the Scherrer constant (equal to 0.94), λ the wavelength of the incident Cu-Kα radiation (1.54059 A), β the line broadening at full width at half maximum, and θ Bragg’s diffraction angle.10)
The calculated average crystallite sizes of the samples are given in Table 1. The Z3T and Z7T nanocomposites showed ZnO crystallite sizes of 26.8 and 26.3 nm, respectively. The crystallite size of the TiO2 nanopowder incorporated in the ZnO sol-matrix was about 9 nm.
3.3. Microstructure of ZnO/TiO2 nanocomposites
The morphology of the ZnO/TiO2 nanocomposites was examined by FE-SEM and TEM. The FE-SEM images of the ZnO/TiO2 nanocomposites calcined at 500°C are shown in Fig. 3. It was found that the ZnO/TiO2 nanocomposite powders formed spherical agglomerates and consisted of rod-like ZnO particles (can be observed clearly from the FE-SEM image of Z5T). Fig. 3 also shows the line-scan mapping results of the nanocomposites, which give an insight into the microstructural composition of the nanocomposites. The EDX analysis results revealed that the ZnO/TiO2 nanocomposites consisted of only Zn, O, and Ti. Following are the atomic ratios in which these elements were present in the nanocomposites: Z3T Zn (77.51), O (21.71), and Ti (0.78); Z7T Zn (63.55), O (23.27), and Ti (13.18).
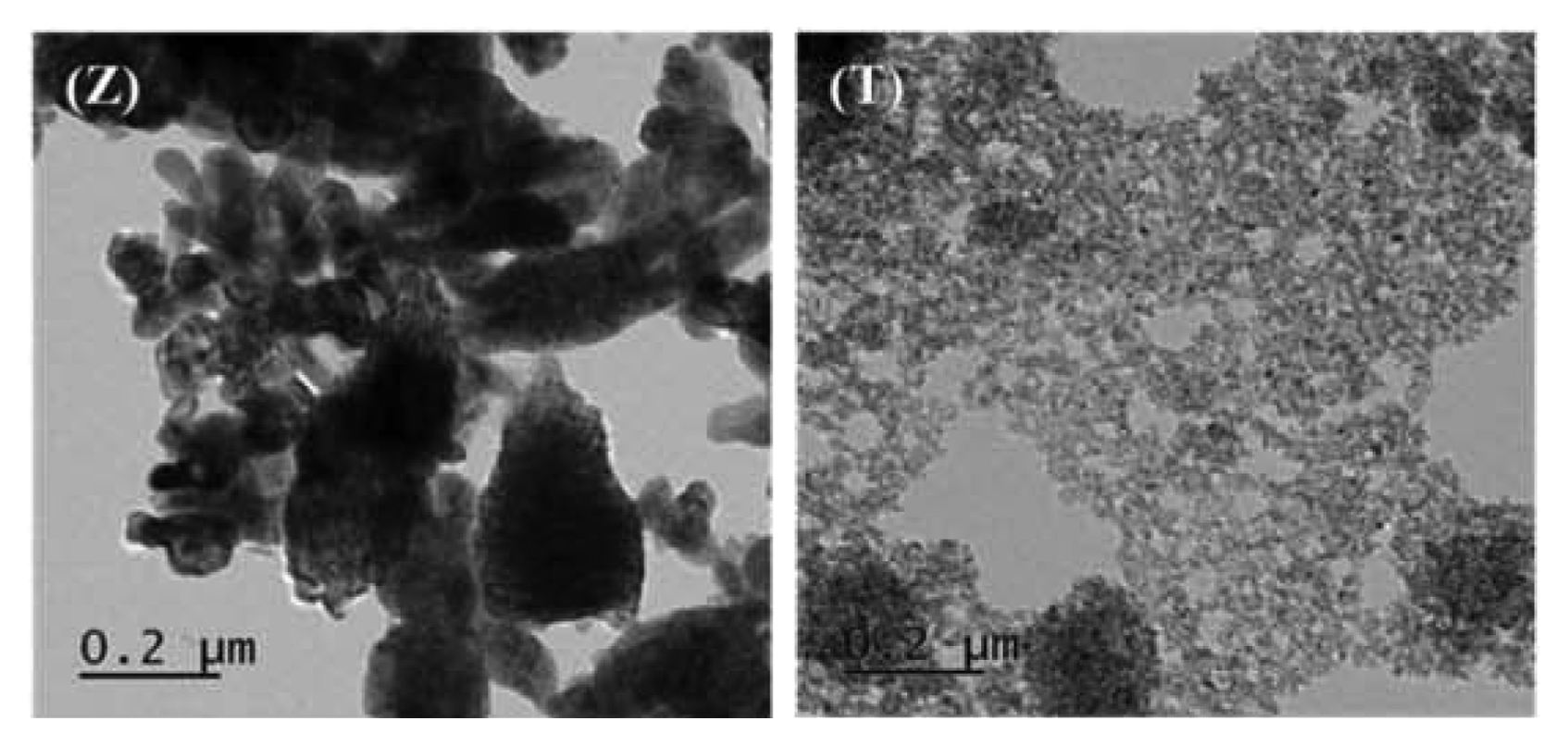
The TEM images of the samples are shown in Figs. 4 and 5. The TEM results showed that the nanocomposites were made up of rod-like ZnO and spherical TiO2 particles. The images revealed that the ZnO and TiO2 particles coexisted in the nanocomposites. It was also observed that some of the TiO2 particles were embedded in the ZnO particles, while some were attached onto the ZnO surface. The images further confirm the rod-like shape of the ZnO particles in the nanocomposites. The TiO2 particles on the other hand were spherical and had very small sizes.
3.4. Optical properties
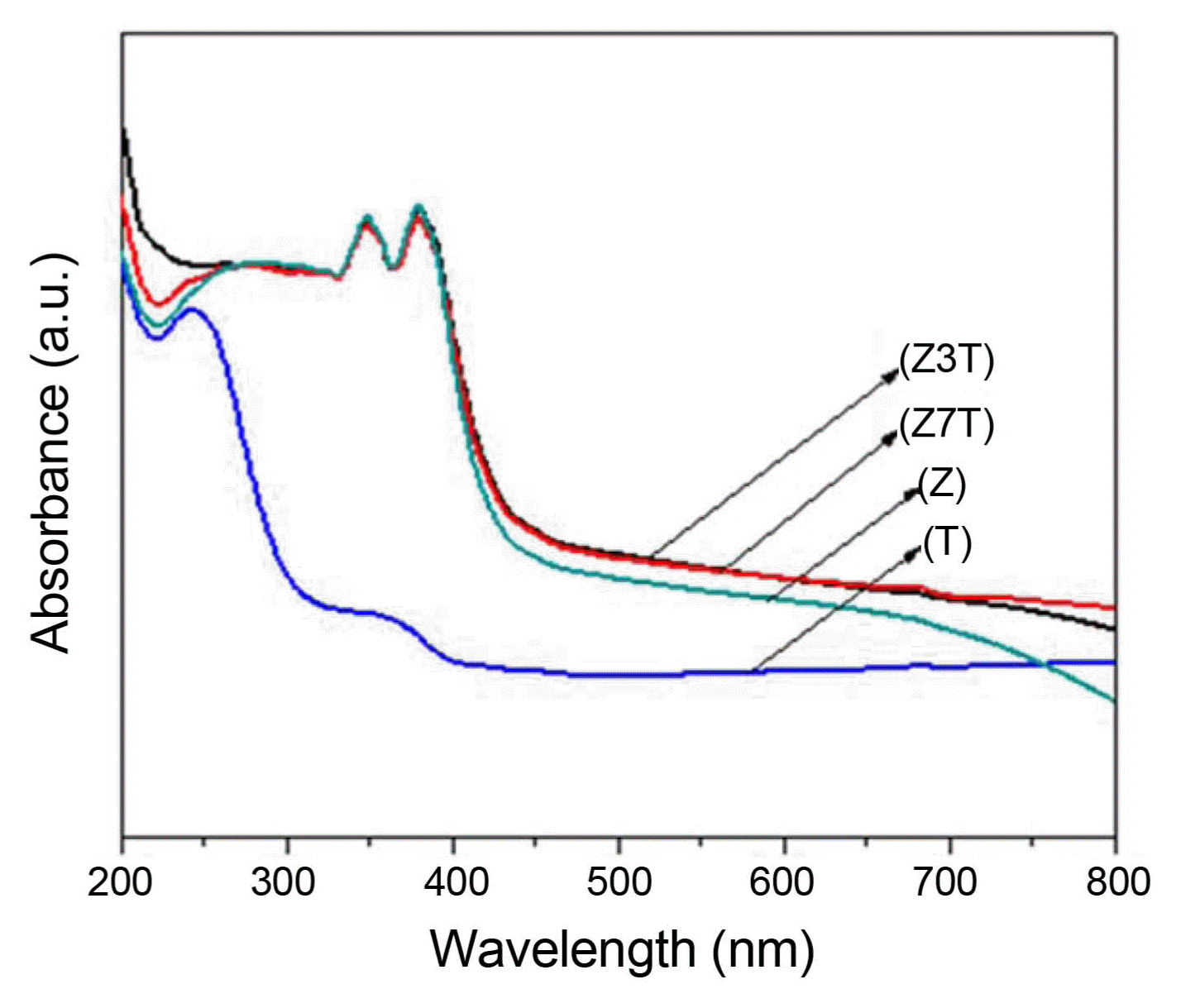
Figure 6 shows the UV-vis absorbance spectra of the calcined samples (pure ZnO and TiO2 and the Z3T and Z7T nanocomposites). Pure TiO2 (anatase) and ZnO have wide bandgaps of 3.2 and 3.37 eV, respectively, which limits their use of the solar spectrum to only UV light (λ ≤ 385 nm).6) The ZnO/TiO2 nanocomposites exhibited enhanced absorbance in the visible light region compared to pure ZnO and TiO2. The ZnO/TiO2 nanocomposites (Z3T, Z7T) showed a strong absorption edge between 380 and 390 nm. However, pure ZnO showed a strong absorption peak between 370 and 380 nm corresponding to ZnO bandgap absorption, while pure TiO2 showed a broad peak at around 360 nm and a strong absorption peak between 240 and 260 nm ascribed to its 3.2 eV photon energy gap.9) The ZnO/TiO2 nanocomposites also showed enhanced light absorption (> 400 nm), while pure ZnO was more right-shifted compared to TiO2. Thus, the combination of ZnO and TiO2 to form ZnO/TiO2 nanocomposites is an effective approach to improving the physicochemical properties of ZnO and TiO2. This is because ZnO and TiO2 have similar photocatalytic mechanisms.4) They also have similar bandgap energies with the energy levels located at almost the same positions. The electron-hole recombination in pure ZnO and TiO2 can also be suppressed by the enhanced charge separation in ZnO/TiO2 composites.4,5) Thus, on the basis of the results obtained in this study, it can be stated that the absorption properties of ZnO/TiO2 nanocomposites can be improved by increasing their TiO2 contents.
4. Conclusions
In this study, we synthesized and characterized ZnO/TiO2 nanocomposites consisting of 30 and 70 wt% TiO2 nanopowder particles embedded in the ZnO sol-matrix. XRD results showed that both the TiO2 anatase and ZnO wurtzite phases were present in the nanocomposites and no impurity compounds were observed. The crystallite sizes of the Z3T and Z7T nanocomposites were found to be 26.8 and 26.3 nm, respectively. SEM results showed that the nanocomposites consisted of spherical TiO2 and rod-like ZnO particles. TEM results confirmed that spherical TiO2 particles were embedded in the lattice. Some of the TiO2 particles were also attached onto the surface of the rod-like ZnO particles. The ZnO/TiO2 nanocomposites exhibited enhanced absorbance (with their light absorption extended to more than 400 nm corresponding to higher wavelengths) compared to that of pure ZnO and TiO2.