1. Introduction
ZnO, which is a II-VI binary compound semiconductor, resides at the borderline between the covalent and ionic semiconductors. The crystal structure of ZnO exhibits the polymorphism of thermodynamically stable hexagonal wurtzite and metastable cubic (zinc blende and rocksalt) phases. ZnO crystallizes in either a cubic zinc blende or a hexagonal wurtzite structure in which each anion of oxygen is surrounded by four cations of zinc at the corners of a tetrahedron, and vice versa.
ZnO can be found in numerous industrial products such as ceramics, catalysts, rubber additives, paints, medical, cosmetics, etc.1) Also, it is used in solar cell windows, piezoelectric transducers, gas sensors, ultraviolet (UV) light emitting/detecting technology and devices with optoelectronic properties.2-6) It has many advanced properties, such as wide band gap energy (3.37 eV) with large exciton binding energy (60 meV), high transparency, very low resistivity with superior electron transport, nontoxicity and abundance in nature.
Recently, the properties of ZnO have been found to depend on its underlying structure, which is considerably dependent on the crystal size and morphology. Various methods have been attempted for the preparation of ZnO crystallites, including the sol-gel method, thermal evaporative decomposition of solutions, wet chemical synthesis, chemical vapor deposition and the hydrothermal method.7-10) However, most of these methods involve high temperatures, high cost and sometimes complicated processes, which might result in impurities in the final products.
In order to obtain ZnO with appropriate chemical and optoelectronic properties for intended applications, control of morphology and particle size are very important. Especially, numerous studies have demonstrated that different morphologies of ZnO such as spherical, rod-like, flower-like, tube, disk and wires can be successfully synthesized by various methods.9-16)
The glycol for the glycol process has multiple functions: it acts as a nonaqueous solvent, stabilizer, and reducing agent; limits particle growth; and prevents agglomeration. The glycol process have proven to be useful in the synthesis of metal sulfide and oxide nanopowders because of their relatively facile and convenient procedures and low reaction temperatures without the need of a mineralizer for processing. In this paper, we present a glycol process to synthesize highly crystallized ZnO nanopowders at low reaction temperature. The effect of a binary solvent of 1,4-butanediol and water on the morphology of ZnO synthesized by glycol process is described, and its properties are characterized.
2. Experimental Procedure
The ZnO nanopowders were synthesized using a glycol process in a glycol solvent. In this study, zinc acetate dihydrate (Zn(CH3COO)2·2H2O) and 1,4-butanediol (C4H10O2) were used as Zn source material and nonaqueous solvent. Various water contents were used as a part of the binary solvent mixtures to control the morphology of the ZnO powders obtained. First, 0.05 mol of zinc acetate dihydrate was dissolved in 100 ml of 1,4-butanediol and in binary solvent mixtures of 100 ml of 1,4-butanediol and various contents of water. The resulting solutions were transferred to a 1000 ml 3 neck-glass container with heating mantle. To investigate the effect of the different synthesis conditions on the phase and morphology of the obtained powders, the glycol reactions were performed at temperatures ranging from 100 to 175°C for 30 min. The content of water at the desired temperature and time was varied between 0 and 30 ml. After the glycol reaction, the glass container was cooled to room temperature. The obtained powders were washed at least 3 times by repeated cycles of centrifugation and redispersion in ethanol and dried at 100°C in a dry oven for 24 h.
The dried powders were analyzed using X-ray diffraction (XRD-D1w, Target: Cu, 30 kV - 30 mA, Shimazu, Japan) over a 2θ range of 10 to 80° with a scanning speed of 2°/min to determine the phase composition and crystal structure. The morphology of the synthesized powders was observed using field emission scanning electron microscopy (FE-SEM, S-4800, Hitachi, Japan) and transmission electron microscopy (TEM, JEM-2100F, JEOL, Japan). The diffuse reflectance spectroscopy (DRS) and photoluminescence (PL) of the ZnO powders were analyzed using a ultraviolet-visible (UV-Vis) spectrometer (JASCO V550, Japan) with BaSO4 as a reference standard and a He-Cd laser with a wavelength of 325 nm as an excitation source at room temperature (LabRAM, Horiba).
3. Results and Discussion
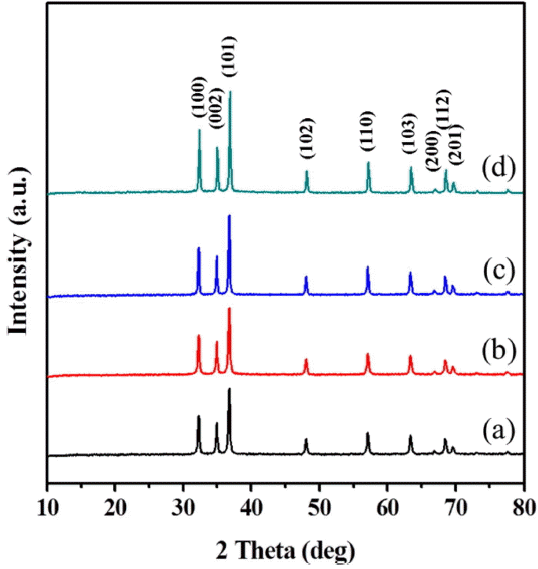
The XRD patterns of each powder obtained by the glycol process at various reaction temperatures for the same reaction time of 30 min are shown in Fig. 1. At the reaction temperature of 100°C, the XRD pattern of the powder shows the peak of crystalline ZnO as the main hexagonal phase and that of poorly crystalline Zn-glycolate as a second phase. Pure ZnO powders of hexagonal structure were prepared without any second phases at temperatures of 125°C and over, and the entire set of peak patterns matches the reference peaks reported on JCPDS Card No. 89-0510. Upon increasing the reaction temperature, the diffraction peaks of ZnO became more intense and narrow, reflecting an increase in the crystallinity of the hexagonal phase. The crystallite size of the ZnO powder was calculated from the broadening of the (101) peak using Scherrer’s equation:
where D is the average crystalline size, λ (= 0.15406 nm) is the wavelength of Cu, β is the full-width at half-maximum, and θ is the diffraction angle of the center of the peak. The crystalline size of the synthesized ZnO nanopowder was observed to be approximately 30 - 40 nm.
FE-SEM images of each powder synthesized at various reaction temperatures for 30 min using the glycol process are presented in Fig. 2. The ZnO powder obtained at the relatively low reaction temperature of 125°C consisted of aggregated secondary powders with spherical morphology and a size of approximately 300 - 500 nm; these powders contained many small primary nanopowders with size of approximately 20 nm, as shown in Fig. 2(a). The SEM images reveal that the size of the ZnO nanopowders increased from 30 to 50 nm when the reaction temperature was increased from 150 to 175°C, as can be observed in Figs. 2(b) and (c). Furthermore, the obtained ZnO nanopowders also exhibited a quasi-spherical morphology without aggregation or morphological change. We suggest that the formation of the aggregate as a spherical powder is the result of the dipole nature of ZnO on the nanometer scale at lower reaction temperatures, as shown in Fig. 2(a). However the aggregation of nanocrystallites decreases with an increase in the temperature because of the flammability and decomposition of 1,4-butanediol at or above the flash point of approximately 134°C.
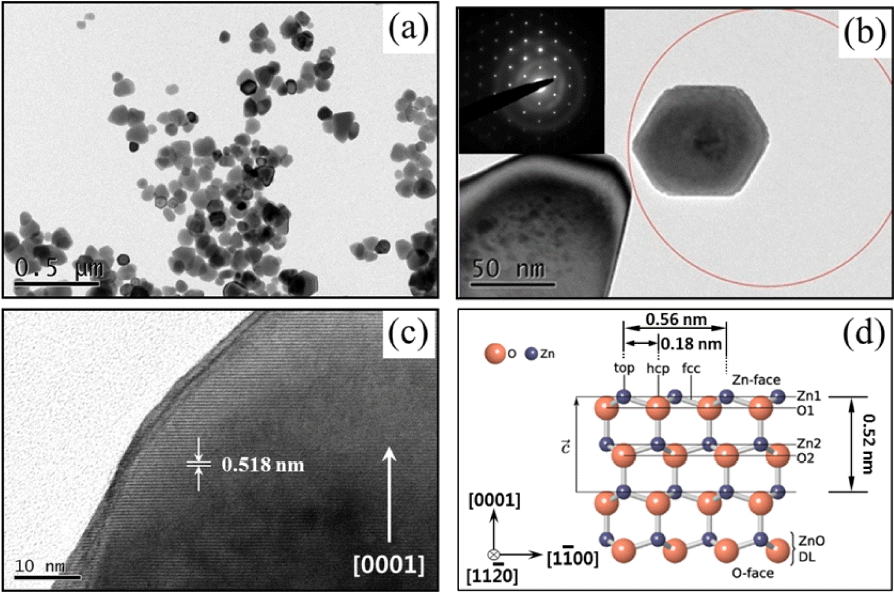
Figure 3 presents a TEM image and corresponding selected-area electron diffraction (SAED) pattern of the ZnO nanopowder synthesized by the glycol process at 150°C for 30 min. The ZnO nanopowders, with quasi-spherical shape, are well dispersed with a relatively narrow size distribution and exhibit a single crystalline nature. The high-resolution TEM image also clearly reveals that the obtained ZnO nanopowders exhibited lattice fringes with an inter-planar distance of approximately 0.28 nm, corresponding to the (1120) plane of the hexagonal structure, as shown in Fig. 3(c). Fig. 3(d) is a schematic illustration showing that the lattice constant of the ZnO structure is very similar to that estimated by analysis of the HRTEM image.
Figure 4 depicts the UV-Vis spectra of the ZnO nanopowders synthesized by glycol process at various reaction temperatures. All of the samples exhibit reflectance thresholds of 382 nm and high reflectance in the visible range.
Here, K is the Kubelka-Munk absorption coefficient, R is the reflectance (%), and hv is the photon energy. According to the plot of [K·hν]2 versus energy, presented in Fig. 4 (inset), the band gap energy (Eg) of the ZnO nanopowder was calculated and found to be 3.25 eV by the extrapolation to [K·hν]2 = 0. This result indicates that a ZnO nanopowder with a wide band gap energy was successfully synthesized.
The effect of water on the morphology of the ZnO powders under glycol reactions of binary solvent mixtures was investigated at 150°C for 30 min. The XRD analysis results for the powders synthesized at different levels of water content (addition of 5 to 30 ml) are given in Fig. 5. Phase-pure ZnO powders with hexagonal structure were synthesized and improved with increasing water content.
Figure 6 shows the powder shape of ZnO synthesized under binary solvent at 150°C for 30 min when the water content increased from 5 ml to 30 ml. The ZnO powders maintained a quasi-sphere shape without morphological change until the addition of 10 ml of the water content. The size of these powders slightly increased from 40 nm to 50 nm, as shown in Figs. 6(a) and (b). When 20 ml of water was added, the morphology of the ZnO powders changed from quasi-spherical to hexagonal plate and pyramid, and the size increased to 100 - 200 nm, as shown in Fig. 6(c). Furthermore, when water was added at a level of more than 30 ml, the shape of the ZnO powder changed to hexagonal prismatic with pyramidal tip and the size of the powders increased drastically to approximately 200 - 500 nm in diameter and 0.5 - 1 μm in length, as shown in Fig. 6(d). Based on the results, it is demonstrated that the size and morphology of ZnO powders can be controlled simply by changing the water content of the binary solvent mixture in the glycol process. The molecule of Zn(CH3COO)2 is imperfectly soluble in 1,4-butanediol and is soluble in water. It is reasonable to assume that the solubility increased with increasing of the water content in the glycol condition of the mixed solvent. As the amount of water increase, more and more of the molecules of Zn(CH3COO)2 dissolve in the 1,4-butanediol and water solution, which might lead to the fast growth of ZnO crystals and result in the formation of polyhedral ZnO hexagonal plates or pyramids and ZnO with a hexagonal prismatic shape and a pyramidal tip.17)
The mechanism of ZnO size control via the changing of the water content was the probably related to the impact of water on the simultaneous course of hydrolysis of Zn(CH3COO)2, as well as being related to the generated intermediates. The OH− ion is supplied by the CH3COO− ion through hydrolysis.18) Zn and O atoms are stacked alternatively along the c-axis, and the top face (0001) consisting of tetrahedral zinc is terminated in OH− ligand due to addition of water. The formation of hexagonal pyramid- and prism-like ZnO crystals is attributed to the difference in the growth velocities of the various crystal facets.19) The (0001) plane disappears due to its high growth velocity, leading to the pointed shape at the end of the c-axis. On the other hand, in the {1011} planes and the {1010} planes, due to slow growth velocity, the crystal remains to form hexagonal pyramid like tips and hexagonal prisms, respectively.
The TEM image and corresponding SAED pattern of the ZnO powders synthesized in glycol condition with 20 ml of water at 150°C for 30 min are shown in Fig. 7. The ZnO powders with hexagonal plate and pyramid shape are well dispersed, have a relatively narrow size distribution, and exhibit a single crystalline nature. Fig. 7(b) shows the HRTEM image of the ZnO nanoplates. These are rather regular hexagon plates; the inset SAED pattern can be indexed as hexagonal ZnO along the [0001] axis; this also proves the single crystal structure. The HRTEM image shown in Fig. 7(c) clearly indicates that the ZnO lattice fringes, with an inter-planar distance of approximately 0.518 nm, correspond to the d spacing of (0001) planes, which reveals that the c-axis orientation is the potential growth direction.
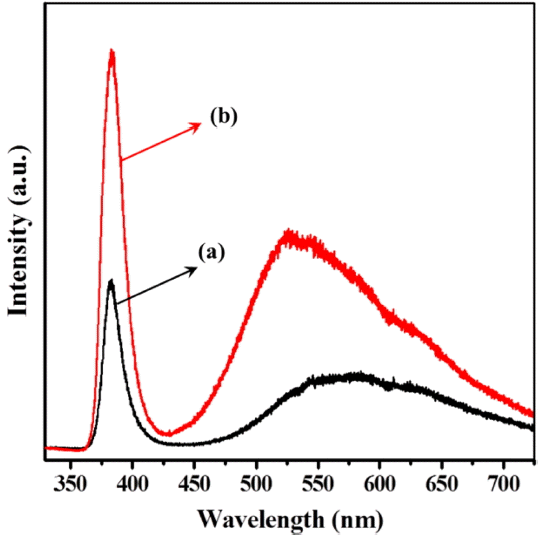
PL spectra of the ZnO nanopowders synthesized by the glycol process were obtained using an He-Cd laser of 325 nm excitation at room temperature; the results are presented in Fig. 8. It can be seen that there are two main emission peaks for both samples: the first are the strong PL peaks at approximately 384 nm, which correspond to the typical ZnO near band edge UV emission with a band gap of 3.23 eV, and which are attributed to the recombination of free excitons. The others are wide band emissions extending from 450 to 700 nm, related to the blue-yellow regions, and are attributed to the presence of ionized oxygen vacancies on the surface and to interstitial Zn ions.20,21) In our results, the strong UV emission should be attributed to the high purity with crystallinity of ZnO nanospheres.
4. Conclusions
In summary, ZnO nanopowders with hexagonal structures were successfully synthesized by glycol process at a temperature above 125°C for 30 min. The quasi-spherical ZnO powder was found to have diameters of approximately 30 - 50 nm without morphological change when glycol was used as a solvent, regardless of the increase of the reaction temperature. With increasing water content, the shape of the ZnO powder changed dramatically from quasi-spherical to hexagonal plate and pyramid, and finally to hexagonal prismatic with a pyramidal tip. The size and morphology of ZnO can be successfully controlled by adjusting the water content of the binary solvent mixture via glycol process. In the PL measurements, all of the samples were found to exhibit a strong exciton-related UV emission with a weak defect peak, suggesting that the ZnO nanopowders achieve their best crystallinity and optical properties under these conditions.