1. Introduction
Rare-earth doped oxides based upconversion (UC) particles are receiving extensive interest in recent years due to their stable luminescent properties and potential for application in products such as lasers, three-dimensional displays, light-emitting devices, solar cells, and biological luminescent imaging.1-3) Previously, scheelite-structured compounds with binary molybdates have been reported to have highly modulated structures due to their unique spectroscopic characteristics and excellent UC photoluminescence properties.4,5) Rare-earth doped binary NaLn(MoO4)2 (Ln = La3+, Gd3+, Y3+) compounds possess a tetragonal structure and space group I41/a; they belong to the family of scheelite-type structures. Trivalent rare-earth ions in tetragonal phase can be partially substituted for by Er3+, Ho3+, Tm3+, and Yb3+ ions. These ions are effectively doped into the crystal lattices of the tetragonal phase because their radii are similar to those of trivalent rare earth ions; such doping results in the excellent UC photoluminescence properties.6-8) Among the rare-earth ions, the Ho3+ ion, due to its appropriate electronic energy level configuration, is suitable for converting infrared to visible light through the UC process. The Yb3+ ion, as a sensitizer, can be dramatically excited by an appropriate incident light source. This energy is transferred to the activator, from which radiation is emitted. The Ho3+ ion activator is the luminescence center of the UC particles, while the sensitizer enhances the UC luminescence efficiency. Co-doping of Ho3+ and Yb3+ ions can remarkably enhance the UC efficiency, allowing a frequency shift from the infrared to the visible range due to the high efficiency of the energy transfer from Yb3+ to Ho3+.9-11)
For the preparation of the binary molybdate NaLn(MoO4)2, several processes have been developed via specific preparation processes, including solid-state reactions,12-15) the sol-gel method,16,17) the Czochralski method,18-21) the hydrothermal method,22-26) the microwave assisted hydrothermal method,27) and the pulse laser deposition.28) It is necessary to create new ternary molybdate compounds for realization of UC photoluminescence in products with such features as well defined morphology and stable UC luminescent properties. However, no NaCaGd(MoO4)3:Ho3+/Yb3+ ternary molybdate phosphors have been reported so far. Compared with the usual methods, microwave synthesis has advantages of very short reaction time, small-size particles, narrow particle size distribution, and high purity of final polycrystalline samples. Microwave heating is delivered to the material surface by radiant and/or convection heating, and this heat energy is transferred to the bulk of the material via conduction.29-31) This is a cost-effective method that can provide products of high homogeneity and can be easily scaled-up; this method is emerging as a viable alternative approach for the quick synthesis of high-quality luminescent materials.
In this way, ternary molybdate NaCaGd (MoO4)3:Ho3+/Yb3+ phosphors synthesized by the microwave sol-gel method are reported for the first time. In this study, ternary molybdate NaCaGd1-x(MoO4)3:Ho3+/Yb3+ phosphors with the proper doping concentrations of Ho3+ and Yb3+ (x = Ho3+ + Yb3+, Ho3+ = 0.05, and Yb3+ = 0.35, 0.40, 0.45, and 0.50) were successfully prepared by the microwave sol-gel method followed by heat treatment. The synthesized particles were characterized by X-ray diffraction (XRD) and scanning electron microscopy (SEM). The pump power dependence of the UC emission intensity and the Commission Internationale de L’Eclairage (CIE) chromatic coordinates were evaluated in detail. The optical properties were examined using photoluminescence (PL) emission and Raman spectroscopy.
2. Experimental Procedure
Stoichiometric amounts of Ca(NO3)2·4H2O (99%, Sigma-Aldrich, USA), Na2MoO4·2H2O (99%, Sigma-Aldrich, USA), Gd(NO3)3·6H2O (99%, Sigma-Aldrich, USA), (NH4)6Mo7O24·4H2O (99%, Alfa Aesar, USA), Ho(NO3)3·5H2O (99.9%, Sigma-Aldrich, USA), Yb(NO3)3·5H2O (99.9%, Sigma-Aldrich, USA), citric acid (99.5%, Daejung Chemicals, Korea), NH4 OH (A.R.), ethylene glycol (A.R.), and distilled water were used to prepare NaCaGd(MoO4)3, NaCaGd0.6(MoO4)3: Ho0.05 Yb0.35, NaCaGd0.55(MoO4)3: Ho0.05Yb0.40, NaCaGd0.50(MoO4)3: Ho0.05Yb0.45, and NaCaGd0.45(MoO4)3:Ho0.05Yb0.50. The compounds Ca(NO3)2, Na2MoO4·2H2O, and (NH4)6Mo7O24·4H2O were dissolved in 20 mL of ethylene glycol and 80 mL of 5M NH4OH under vigorous stirring and heating. Subsequently, Gd(NO3)3·6H2O with Ho(NO3)3·5H2O, Yb(NO3)3·5H2O, and citric acid (with a molar ratio of citric acid to total metal ions of 2:1) were dissolved in 100 mL of distilled water under ibid. Then, the solutions were mixed together vigorously and were heated at 80 - 100°C. Finally, highly transparent solutions were obtained and adjusted to pH = 7 - 8 by the addition of NH4OH or citric acid. The transparent solutions were placed into a microwave oven operating at a frequency of 2.45 GHz, with maximum output-power of 1250 W for 30 min. The working cycle of the microwave reaction was controlled very precisely using a regime of 40 s on and 20 s off for 15 min; this was followed by further treatment of 30 s on and 30 s off for 15 min. The samples were treated with ultrasonic radiation for 10 min to produce light-yellowish transparent sols. After this, the sols were dried at 120°C in a dry oven to obtain black dried gels. The black dried gels were ground and heat-treated at 900°C for 16 h at 100°C intervals between 600-900°C. Finally, white particles were obtained for pure NaCaGd(MoO4)3 and pink particles were obtained for the doped compositions.
The crystal structure of the synthesized particles was identified using XRD (D/MAX 2200, Rigaku, Japan). The microstructure and surface morphology of the synthesized particles were observed using SEM (JSM-5600, JEOL, Japan). The PL spectra were recorded using a spectrophotometer (Perkin Elmer LS55, UK) at room temperature. The pump power dependence of the UC emission intensity was measured at levels of working power from 20 to 110 mW. Raman spectra measurements were performed using a LabRam Aramis (Horiba Jobin-Yvon, France) with a spectral resolution of 2 cm−1. The 514.5-nm line of an Ar ion laser was used as an excitation source; the power on the samples was kept at a 0.5 mW level to avoid sample decomposition.
3. Results and Discussion
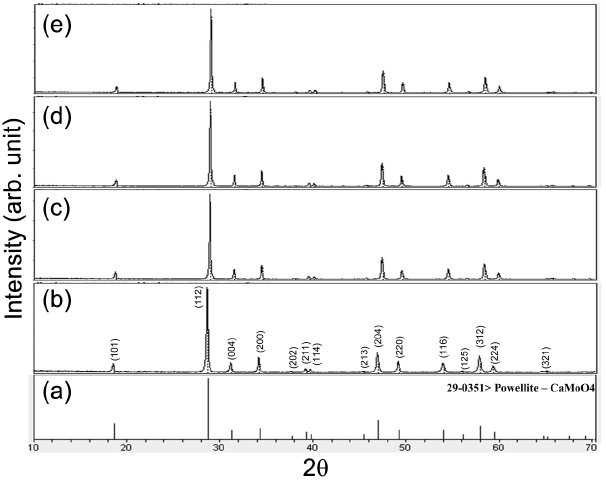
Figure 1 shows XRD patterns of the (a) JCPDS 29-0351 pattern of powellite CaMoO4, the synthesized (b) pure NaCa Gd(MoO4)3, (c) NaCaGd0.6(MoO4)3:Ho0.05Yb0.35, (d) NaCaGd0.55 (MoO4)3:Ho0.05Yb0.40, (e) NaCaGd0.50(MoO4)3:Ho0.05Yb0.45, and (f) NaCaGd0.45(MoO4)3:Ho0.05Yb0.50 particles. In the synthesized samples, almost all XRD peaks can be assigned to the tetragonal phase, which is consistent with the standard data for CaMoO4 (JCPDS 29-0351). The sites of Ca2+ or (Na+/Gd3+) ions were taken as occupied by Ca2+, Na+, Gd3+, Ho3+, and Yb3+ ions at fixed occupation levels as determined by the nominal compositions. The Ho3+ and Yb3+ ions can be partially doped into the Gd3+ sites, so that the Gd3+ ions can be efficiently substituted into the Ca2+ sites. Consequently, Na+, Gd3+, Ho3+, and Yb3+ ions can be efficiently substituted into the Ca2+ sites in the NaCaGd(MoO4)3 lattice. The ternary undoped and doped NaCaGd1-x(Ho,Yb)x(MoO4)3 crystallites provide the strongest intensity peaks from the (112), (204), and (312) planes; these are the major peaks of CaMoO4. Therefore, the synthesized NaCaGd1-x(MoO4)3 particles belong to the molybdate family and have a scheelite-type structure, with space group I41/a. It is observed that the diffraction peaks of the ternary undoped and doped NaCaGd1-x(MoO4)3 samples in Fig. 1(b)-(f) shift slightly to higher angles compared to that of the standard data for CaMoO4 (JCPDS 29-0351) shown in Fig. 1(a). It is well known that the relationship of the interplanar space (dhkl), diffraction angle (θ), and wavelength of X-rays (λ) can be expressed using Bragg’s equation:
In pure NaCaGd(MoO4)3 crystals, unit cell decrease occurs due to the substitution of Na+ (R=1.18 Å) and Gd3+ (R=1.053 Å) ions into the Ca3+ (R=1.12 Å) sites.32) According to the Bragg equation (1), the diffraction peaks shift to higher angles with the decrease of the dhkl values. It can also be observed that the diffraction peaks of the doped samples in Fig. 1(c)-(f) shift slightly to higher angles compared to that of the pure NaCaGd(MoO4)3 sample shown in Fig. 1(b). In the crystal structure of NaCaGd1-x(MoO4)3, the Gd3+ ion site is supposed to be occupied by Ho3+ and Yb3+ ions with occupations fixed according to the nominal chemical formulas. The defined crystal structure contains MoO4 tetrahedrons coordinated by four (Ca/Na/Gd/Ho/Yb)O8 square antiprisms through the common O ions. In the doped crystals, unit cell shrinkage results from the substitution of Gd3+ ions by Ho3+ and Yb3+ ions. It is assumed that the radii of Ho3+ (R=1.015 Å) and Yb3+ (R=0.985 Å) are smaller than that of Gd3+ (R=1.053 Å) when the coordination number is CN = 8.32) Consequently, it should be emphasized that the Ho3+ and Yb3+ ions can be efficiently doped into the NaCaGd(MoO4)3 lattice by partial substitution of Ho3+ and Yb3+ for Gd3+ in the Gd3+ sites, which leads to unit cell shrinkage while the tetragonal structure of NaCaGd1-x(Ho,Yb)x(MoO4)3 maintains. Post heat-treatment plays an important role in establishing a well-defined crystallized morphology. To reach a well-defined final morphology, the samples need to be heat treated at 900°C for 16 h. It is assumed that the Ho3+/Yb3+ doping concentrations are acceptable to keep the original structure of NaCaGd(MoO4)3.
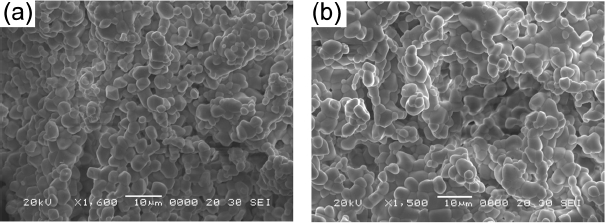
Figure 2 provides SEM images of the synthesized (a) NaCaGd0.6(MoO4)3:Ho0.05Yb0.35 and (b) NaCaGd0.50(MoO4)3:Ho0.05 Yb0.45 particles. The as-synthesized samples are well crystallized, with a fine and homogeneous morphology and a particle size of 3-5 μm. The samples show no discrepancy in terms of morphological features, and agglomerated particles are induced by atom inter-diffusion between the grains. It should be noted that the morphological feature is insensitive to the Ho3+/Yb3+ doping concentration. The microwave sol-gel method in application to ternary molybdates provides the energy to synthesize the bulk of the material uniformly, so that fine particles with controlled morphology can be fabricated in a short time period. This method is a cost-effective way to fabricate highly homogeneous products with easy scale-up; it is a viable alternative to the rapid synthesis of UC particles. This suggests that the microwave sol-gel route is suitable for the creation of homogeneous NaCaGd1-x(MoO4)3:Ho3+/Yb3+ crystallites.
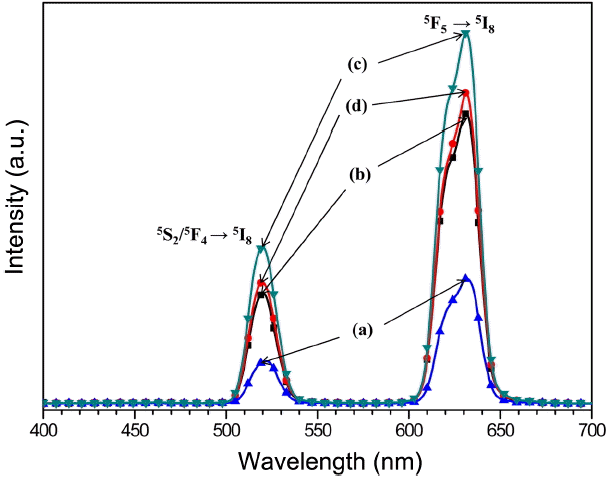
Figure 3 shows UC photoluminescence emission spectra of the as-prepared (a) NaCaGd0.6(MoO4)3:Ho0.05Yb0.35, (b) Na CaGd0.55(MoO4)3:Ho0.05Yb0.40, (c) NaCaGd0.50(MoO4)3:Ho0.05Yb0.45, and (d) NaCaGd0.45(MoO4)3:Ho0.05Yb0.50 excited under 980 nm at room temperature. The UC particles exhibited yellow emissions based on a strong 520 nm emission band in the green region and a strong 630-nm emission band in the red region. The UC intensity of (c) NaCaGd0.50(MoO4)3:Ho0.05 Yb0.45 is the highest of all particles. The strong 520-nm emission band in the green region corresponds to the 5S2/5F4 → 5I8 transition, while the very strong 630-nm emission band in the red region corresponds to the 5F5 → 5I8 transition to the activator, where radiation is emitted. The Ho3+ ion activator is the luminescence center for these UC particles; the sensitizer Yb3+ effectively enhances the UC luminescence intensity because of the efficient energy transfer from Yb3+ to Ho3+. As can be seen in Fig. 3, the highest intensity of (c) NaCaGd0.50(MoO4)3:Ho0.05Yb0.45 resulted for the ratio of Yb3+: Ho3+ 9:1, while the lowest intensity of (a) NaCaGd0.6 (MoO4)3: Ho0.05Yb0.35 for the ratio of Yb3+:Ho3+ 7 : 1. The optimal Yb3+:Ho3+ ratio 9 : 1 is induced by the concentration quenching effect of the Ho3+ ion. Therefore, the higher content of the Yb3+ ion, used as a sensitizer, and the lower content of the Ho3+ ion, used to ensure the correct ratio of Yb3+:Ho3+ (9 : 1), can remarkably enhance the UC luminescence through efficient energy transfer. The concentration quenching effect can be explained by the energy transfer between nearest Ho3+ and Yb3+ ions. With the increase of the Ho3+ and Yb3+ ion concentrations, the distance between Ho3+ and Yb3+ ions decreases, which can promote non-radiative energy transfer via an exchange interaction or multipole-multipole interactions.33)
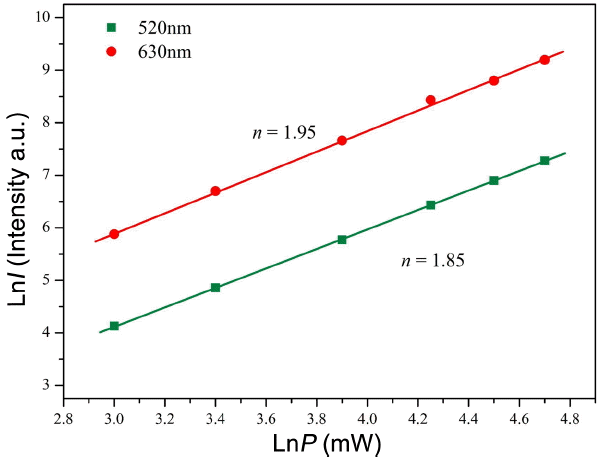
The logarithmic scale dependence of the UC emission intensities at 520 and 630 nm on the working pump power over the range of 20 to 110 mW in the NaCaGd0.50(MoO4)3: Ho0.05Yb0.45 sample is shown in Fig. 4. In the UC process, the UC emission intensity is proportional to the slope value n of the irradiation pumping power, where n is the number of pumped photons required to produce UC emission:34)
where the value n is the number of pumped photons required to excite the upper emitting state, I is the UC luminescent intensity, and P is the laser pumping power. As is evident in Fig. 4, the slope value calculations indicate a value of n = 1.95 for the red emission at 630 nm, and a value of n = 1.85 for the green emission at 520 nm. These results show that the UC mechanism of the green and red emissions can be explained by a two-photon UC process in Ho+3/Yb3+ co-doped phosphors.4,9-12)
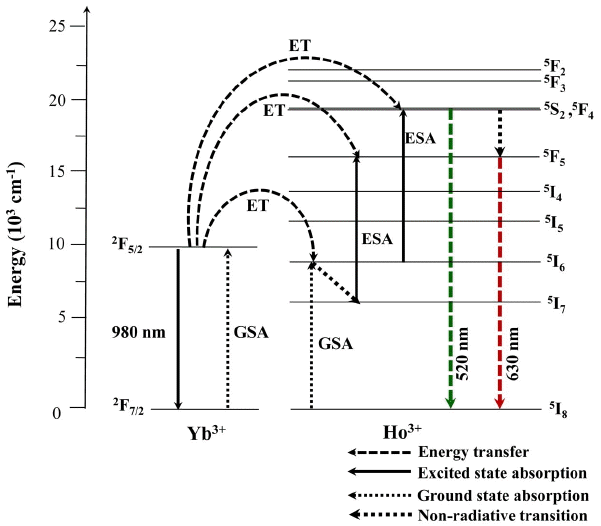
Based on the results of pump power dependence, the known schematic energy level diagrams of Ho3+ (activator) and Yb3+ (sensitizer) ions in the as-prepared NaCaGd1-x (MoO4)3:Ho3+/Yb3+ samples, and the UC mechanisms, which account for the green and red emissions during 980 nm laser excitation, are shown in Fig. 5. The UC emissions are generated by a two photon process through excited state absorption (ESA) and energy transfer (ET).4,10,12) Initially, the Yb3+ ion sensitizer is excited from the 2F7/2 level to the2F5/2 level under excitation by 980 nm pumping; the Yb3+ ion sensitizer transfers its energy to the Ho3+ ions. Then, the Ho3+ ions are populated from the 5I8 ground state to the 5I6 excited state. This is a phonon-assisted energy transfer process because of the energy mismatch between the 2F5/2 level of Yb3+ and the 5I6 level of Ho3+. Second, the Ho3+ in the 5I6 level is excited to the 5S2 or 5F4 level by the next energy transfer from Yb3+. In addition, the 5S2 /5F4 level of Ho3+ can be populated through excited state absorption. Finally, the green emission around 520 nm, corresponding to the 5S2/5F4→5I8 transition, takes place. For the red emission, the population of the 5F5 level is generated by two different channels. In one channel, the Ho3+ in the 5S2/5F4 level state relaxes non-radiatively to the 5F5 level. Another channel is closely related to the 5I7 level, populated by non-radiative relaxation from the 5I6 excited state. The Ho3+ in the 5I7 level is excited to the 5F5 level by energy transfer from Yb3+ and relaxes to the 5F5 level. Therefore, the red emission around 630 nm corresponds to the 5F5 →5I8 transition.35,36)
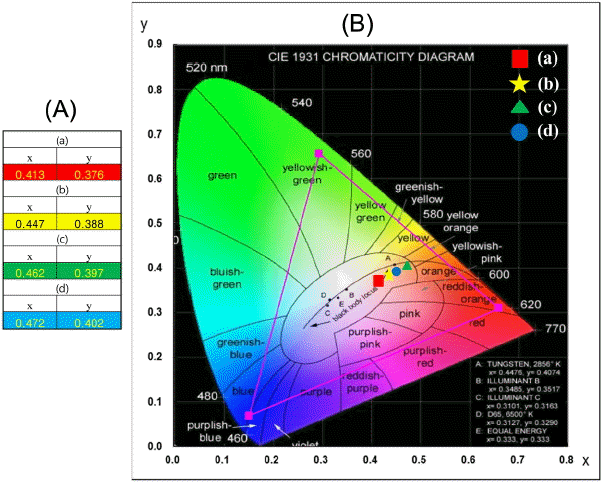
In Fig. 6, (A) calculated chromaticity coordinates (x, y) and (B) a CIE chromaticity diagram are shown for the compositions (a) NaCaGd0.6(MoO4)3:Ho0.05Yb0.35, (b) NaCaGd0.55 (MoO4)3:Ho0.05Yb0.40, (c) NaCaGd0.50(MoO4)3:Ho0.05Yb0.45, and (d) NaCaGd0.45(MoO4)3:Ho0.05Yb0.50. The triangle in Fig. 6(B) indicates standard coordinates for blue, green, and red colors. The inset in Fig. 6(B) shows the chromaticity points for the samples (a), (b), (c), and (d). The chromaticity coordinates (x, y) are strongly dependent on the Ho3+/Yb3+ concentration ratio. As can be seen in Fig. 6(A), the calculated chromaticity coordinates x = 0.413 and y = 0.376 for (a) NaCaGd0.6(MoO4)3: Ho0.05Yb0.35, the values of x = 0.447 and y = 0.388 for (b) NaCa Gd0.55(MoO4)3:Ho0.05Yb0.40, the values of x = 0.462 and y = 0.397 for (c) NaCaGd0.50(MoO4)3:Ho0.05Yb0.45, and the values of x = 0.472 and y = 0.402 for (d) NaCaGd0.45(MoO4)3:Ho0.05Yb0.50 correspond to the standard equal energy points in the CIE diagram shown in Fig. 6(B).
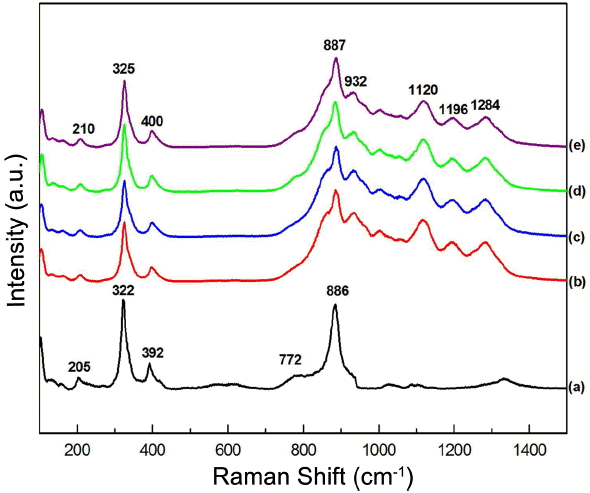
Figure 7 shows the Raman spectra of the synthesized (a) pure NaCaGd(MoO4)3, (b) NaCaGd0.6(MoO4)3:Ho0.05Yb0.35, (c) NaCa Gd0.55(MoO4)3:Ho0.05Yb0.40, (d) NaCaGd0.50(MoO4)3:Ho0.05Yb0.45, and (e) NaCaGd0.45(MoO4)3:Ho0.05Yb0.50 particles excited by the 514.5-nm line of an Ar ion laser at 0.5 mW. The internal modes for the (a) pure NaCaGd(MoO4)3 particles were detected at 126, 205, 322, 392, 772, and 886 cm−1. The well-resolved sharp peaks for NaCaGd(MoO4)3 indicate the high crystallinity of the synthesized particles. The internal vibration mode frequencies are dependent on the lattice parameters and on the strength of the partially covalent bond between the cation and the molecular ionic group MoO4. The Raman spectrum of the NaCaGd(MoO4)3 crystal in Fig. 7(a) is that of the typical molybdate compounds, and is divided into two parts, with a wide empty gap of 400-750 cm−1.4,37,38) The highest intensity of the wavenumber band at 886 cm−1 corresponds to stretching vibrations of MoO4. Stretching vibrations of the Mo-O bonds are observed in the 772 cm−1 region. For these stretching vibrations, strong mixing occurs between the Mo-O bonds and the MoO4. The bands at 322 and 392 cm−1 may have originated from vibrations of the longer Mo-O bonds, which are employed in the formation of the Mo-Mo bridge. Translational vibration motion of the Ca3+ ions is observed at 205 cm−1, whereas the Gd3+ translations are located below 180 cm−1.39,40) The Raman spectra of the doped particles indicate the presence of highly modulated peaks at higher frequencies of 887, 932, 1120, 1196, and 1284 cm−1 and at lower frequencies of 210, 325, and 400 cm−1. It should be emphasized that these strong modulated peaks of the doped samples recorded under excitation at 514.5 nm are superimposed due to strong Ho3+ luminescence lines and strongly affected by the concentration quenching effect of Ho3+ ions.41,42) These results lead to high emitting efficiency; the involved materials can be considered as potential active components in new optoelectronic devices and biomedical applications.
4. Conclusions
Ternary molybdate NaCaGd(MoO4)3:Ho3+/Yb3+ phosphors were successfully synthesized by microwave sol-gel method. Well-crystallized particles formed after heat-treatment at 900°C for 16 h showed fine and homogeneous morphology, with particle sizes of 3 - 5 μm. Under excitation at 980 nm, the doped NaCaGd1-x(MoO4)3:Ho3+/Yb3+ particles exhibited yellow emissions based on a strong 520-nm emission band in the green region and a very strong 630-nm emission band in the red region; these emissions were assigned to the 5S2/5F4→5I8 and 5F5→5I8 transitions, respectively. The optimal Yb3+:Ho3+ ratio was found at 9 : 1, and was determined to be controlled by the concentration quenching effect of the Ho3+ ion. The slope value calculations indicate a value of n = 1.95 for the red emission at 630 nm, and a value of n = 1.85 for the green emission at 520 nm. The calculated chromaticity coordinates were found to correspond to the standard equal energy point in the CIE diagram. The highly modulated Raman spectra of the doped samples, recorded under excitation at 514.5 nm, were superimposed due to strong Ho3+ luminescence lines and strongly affected by the concentration quenching effect of Ho3+ ions. These results can be considered as indicating that these materials have the potential for use as active components in new optoelectronic devices and biomedical applications.