1. Introduction
Titanium dioxide is one of the most commonly used ceramic materials owing to its outstanding physical and chemical properties for both structural and functional engineering applications. Titanium dioxide exhibits a high refractive index (> 2.6), high brightness, and good opacifying properties. Therefore, it has been used as a vivid colorant in a wide variety of products, including paints, paper, plastics, printing inks, fibers, and rubber.1,2) It is employed in inert pigments that are used as colorants in pharmaceutical products and foods (E 171).3-4) Additionally, as an effective UV filter, it is suitable for use in different personal care products and cosmetics such as sunscreens, toothpastes, lipsticks, and pressed and loose powders.1,2) The nontoxic nature and biocompatibility of titanium dioxide render it suitable for utilization in medical applications.5)
Titanium dioxide exists in three polymorphs, namely anatase, rutile, and brookite.6) The most common phases are anatase and rutile, as brookite is thermodynamically less stable. Titanium dioxide is the most widely studied semiconductor material for energy and environmental applications. Nanosized anatase-type TiO2 shows the most effective photocatalytic properties. Owing to this reason, it is extensively applied in self-cleaning materials and water and air purification.7,8,9) In various thermal spray coating applications, TiO2 is used in severe service conditions owing to its excellent hardness, wear resistance, corrosion resistance, chemical stability, and biocompatibility.10) Recent studies also showed that thermally spray coated TiO2 is very useful in the decomposition of environmental water and air pollutants.11) Moreover, TiO2 can be used as a semiconductor metal oxide for the development of gas sensors.12) It also plays an important role in the energy domain such as in the manufacturing of dye-sensitized solar cells and batteries, water splitting, and electrochromic and hydrogen storage.13,14)
The final properties of TiO2 products are dependent on the initial powder size, surface properties, morphology, and crystalline phase.15) Several studies have indicated that the improved efficiency of the photocatalytic process is attributed to the large specific surface area of TiO2 nanoparticles.16) Gell et al. pointed out that nanosized particles enable thermal spray coatings that exhibit different architectures and properties to be fabricated.17) Additionally, particle size also affects the final performance of TiO2 in the fields of hue and hiding and powder metallurgy processing.
However, the use of nanosized powders might cause some fabrication problems because of their low flowability and compactibility.18,19) The reason for that is fine particles tend to form agglomerates owing to their high surface energy. Besides, working with nanosized particles might present a health hazard, and numerous studies have reported that oral exposure to TiO2 nanopowders is harmful to human health3-4) For the aforesaid reasons, controlled agglomeration of fine powders is a commonly used solution in the industry.
Spray drying is a powder processing technique that is widely used for the production of granules.20,21) In spray drying process, a feed slurry transforms into fine droplets by passing through a nozzle, then the atomized droplets are dried by a hot atmosphere that the drying chamber provides.22,23) The granulation of fine powders permits manipulation of the desired properties of powders, which include improved flowability, solubility, density, compactibility, dispersibility, and sinterability. Granulation can be used to prevent dusting that might cause health hazards.24) In the production of granules, preparation of the feed slurry is a key step.17) The characteristics of the suspension determine the morphology of the granules and, subsequently, the properties of the final ceramic product.18,25,26) Other important parameters affecting the obtained granule properties (such as morphology, size, and shape) are the device processing conditions and the characteristics of the starting powder.25-27)
It is apparent from the above discussion that granulation of individual particles or their mixtures to obtain different compositions has drawn much research attention for many engineering applications. TiO2 is employed in the particle form in a wide range of engineering applications where fine powders with high surface areas are desirable. These fine powders can be granulated by different methodologies to eliminate the negative effects mentioned above. In order to optimize the granulation behavior of TiO2 ceramic powders during spray drying, the effects of solid content and the binder/powder ratio in the slurry, solid/liquid ratio, and the amount of additive (polyvinyl alcohol; PVA) on the granulation behavior of TiO2 ceramic powders were investigated in this study.
2. Experimental Procedure
2.1. Starting materials and their characterization
In this study, analytical grade TiO2 (99% purity, Alfa AesarTM) powder and liquid PVA (Optapix PAF35/Zschimmer&Schwarz) were used. Before the fabrication of granules, characterization of the commercial TiO2 starting powders was performed. Their morphology was investigated by scanning electron microscopy (SEM; Jeol-JSM-T330). Particle size and size distribution measurements were carried out by using a Microtrac Nano-flexTM in-situ particle size analyzer. Phase analysis was carried out using the X-ray diffraction method (Bruker D8 Advance X-ray diffractometer) with CuKα radiation (λ = 1.540 Å) from 10° to 80° at a scanning speed of 1°/min. The actual densities of the starting powders were measured by using a gas pycnometer (Micromeritics, model Accupyc2-1340). The specific surface areas of the powders were determined based on the Brunauer-Emmett-Teller (BET) theory with the help of a BET Quanthachrome ™ Autosorb device. Before the BET analysis, the humidity of the commercial powder was removed under vacuum at 200°C for 2 h. Nitrogen (N2) gas was used as an adsorber. The equivalent particle size (DBET) was calculated from the BET surface area results by assuming that all the particles exhibit the same spherical shape and size.28,29)
2.2. Slurry preparation
To investigate the effects of solid content and the binder/powder ratio in the feedstock slurry on the granulation behavior of TiO2, the feed compositions were designed with respect to the solid/liquid ratio (30 wt.%, 35 wt.%, and 40 wt.% solid) and the amount of the additive PVA (2 wt.%, 2.5 wt.%, 3 wt.%, and 3.5 wt.% PVA). All the compositions and sample codes are listed in Table 1.
A calculated amount of liquid PVA as a binder was first added to distilled water and stirred for 30 min at 80°C with a magnetic stirrer until the PVA completely dissolved. Then, TiO2 powder was leisurely added to the suspension and the mixture kept stirring for an additional 30 min. Subsequently, to obtain a more homogenous slurry, the mixture was milled using a planetary ball mill instrument for one hour by using a 250 ml polymeric pot and zirconia balls at a rotation speed of 300 rpm. Approximately 2/3rd of the volume of the pot was filled with the starting materials and grinding media. To obtain a feed slurry with 30 wt.% solid loading and 2 wt.% PVA, 2 g of PVA, 70 ml of distilled water, 30 g of TiO2 powder, and 60 g of zirconia balls were fed into the 250 ml pot. For the preparation of all the feedstock suspensions, the aforesaid procedure was applied.
2.3. Spray drying and characterization of granules
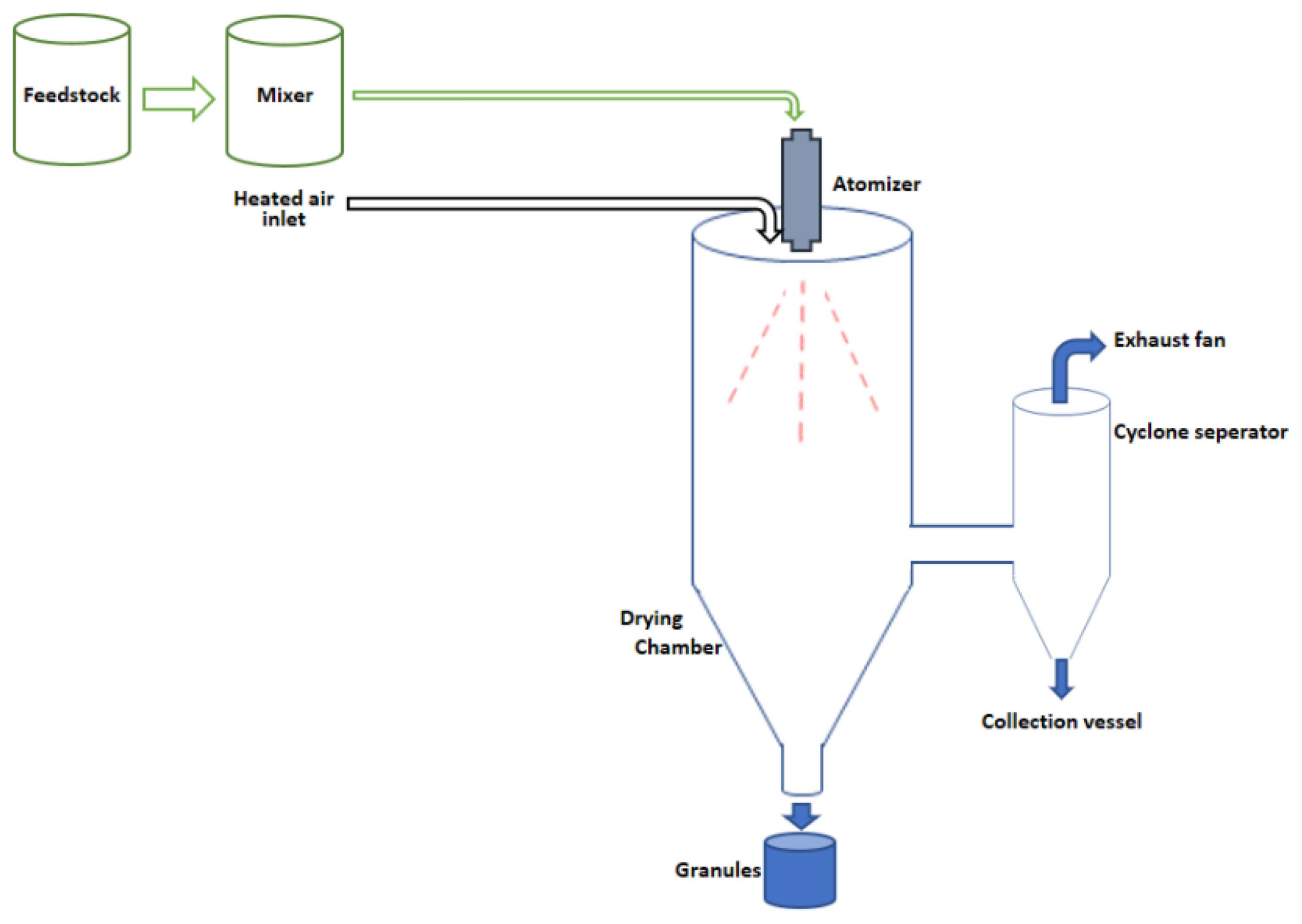
The slurries of different compositions obtained after mixing were granulated by using a BuchiTM Mini Spray Dryer (B290) at 15% flow rate and 100% extractor fan rate. In this study, air was selected as the drying medium. The air temperatures at the inlet and outlet of the drying apparatus were 200°C and 100°C, respectively. These spray dryer parameters were kept constant in all the experiments and the slurries were continuously mixed by magnetic stirrers during the feeding process to prevent sedimentation. The slurry was atomized into small droplets by using a nozzle and the droplets encountered hot air in the drying chamber; they dried as they passed through the chamber. Schematic illustration of the centrifugal spray drying apparatus used in this study is shown in Fig. 1.
The actual densities of the granules produced via spray drying were measured using a gas pycnometer. SEM was used to examine the microstructure of the granules. Image-J (version 1.51h)30) software, which is freely available in the public domain, was used to analyze the average size and size distribution of the granules from the SEM images. For each sample, more than 150 granule sizes were measured. Only those granules that had formed successfully were taken into consideration.
3. Results and Discussion
3.1. Characterization of the starting raw material
The particle size distribution of commercial TiO2 powders is shown in Fig. 2. To obtain a more accurate result, the experiments were repeated three times, and the average measured value has been reported in this paper. The mean particle size of the TiO2 powder was measured as 292.3 nm. According to the particle size distribution, the majority of the powder particles reveal sizes between 100 nm and 1 μm. In addition, a small amount of agglomerated powder that is oversized (~ 3 μm) compared to the majority of the batch was detected in the form of a second peak. Based on BET analysis, the specific surface area of the TiO2 powder (DEBT) is 6.84 m2/g. Furthermore, the equivalent particle size, calculated using equation 1, is found to be 266.6 ± 3 nm. In this equation, δ represents the theoretical density (g/cm3) of the powder. In the calculation, the theoretical density of TiO2 (anatase; 3.78 g/cm3) was used.
The secondary electron images of the commercial TiO2 powder obtained by SEM at different magnifications are displayed in Fig. 3(a) and 3(b). They show that the particles exhibit a uniform equiaxed morphology. According to the SEM observations, the powder reveals a narrow grain size distribution.
X-ray diffraction pattern (XRD) of the commercial TiO2 powder used in this study is shown in Fig. 4. It turns out that the as-received TiO2 powder is composed of anatase phase, without any other phases.
3.2. Granulation behavior of the TiO2 powders
The secondary electron SEM images of all the synthesized granules are shown in Figs. 5, 6, and 7. As can be observed, the granules are generally formed in spherical shapes for all the investigated compositions. This result is thought to be ascribed to the rheological properties of the feedstock slurries. It is known that during the spray drying process of partially coagulated slurries, water moves from the inside of the droplet to its surface and does not allow particles to form a crust on the surface of the droplet, which results in the formation of partially filled round granules. On the contrary, the granules prepared from very well-dispersed feed slurries are known to form donuts via explosion due to the high internal pressure after the material has been transported from the droplet center to its surface during drying.31,32)
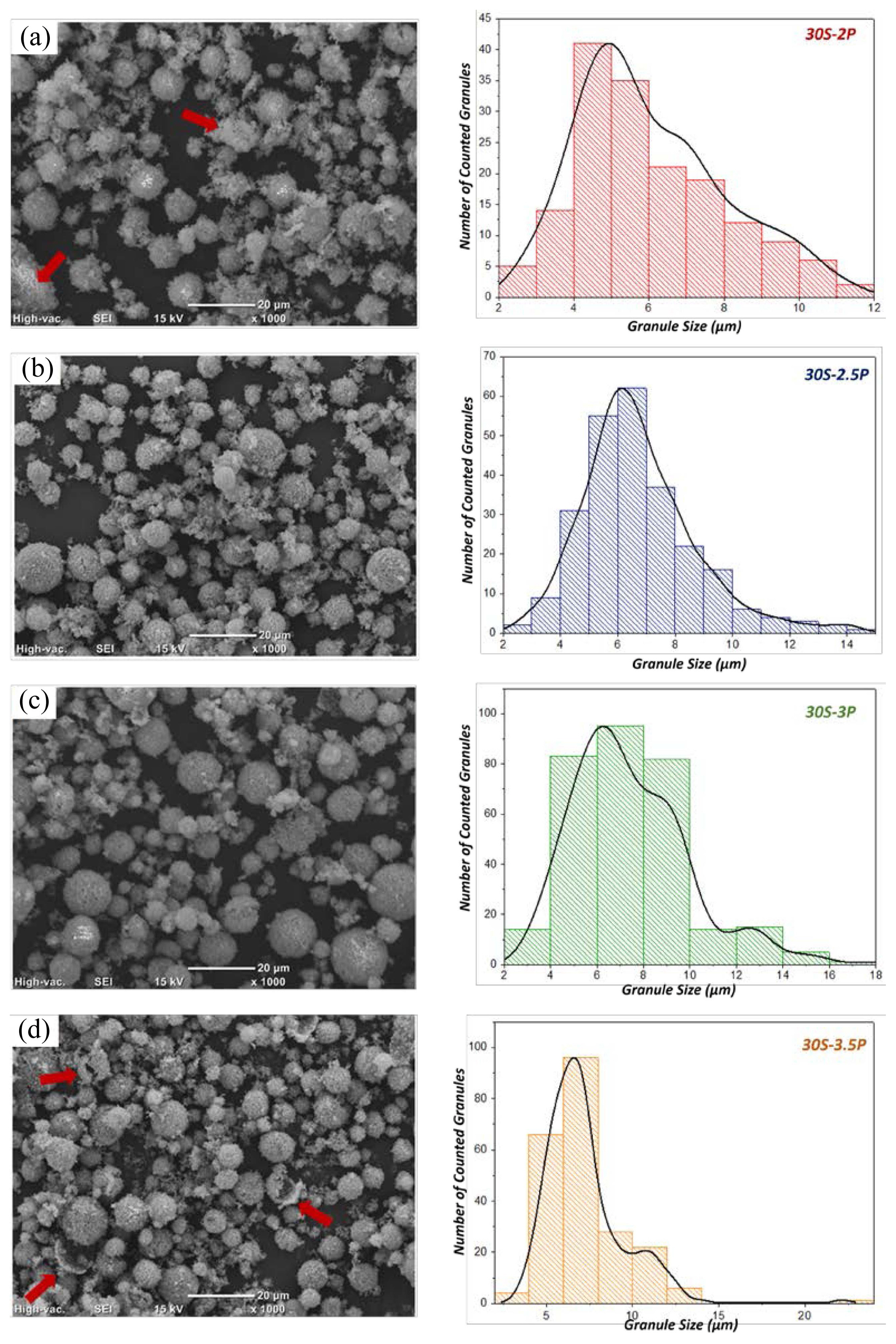
According to the Image-J analysis results, for all the compositions considered, the granule size distribution is fairly wide, 1-20 μm. The grain size distribution of each of the considered powders can be seen in Fig. 5, 6, or 7, beside the related SEM image.
When the SEM images of the samples containing different amounts of PVA are compared, it is seen that when the amount of PVA in the feed composition increases, the size of the granules also tends to increase. The granules prepared from slurries with 3-3.5 wt.% PVA (Figs. 5(c), (d), 6(c), (d), and 7(c), (d)) reveal slightly bigger sizes than those obtained from slurries with 2-2.5 wt.% PVA (Figs. 5(a), (b), 6(a), (b), and 7(a), (b)). Additionally, it is observed that the sample produced from the feed with 2 wt.% PVA contains an excess amount of non-granulated particles and some poorly shaped/broken granules. This phenomenon is thought to occur because of the low PVA content (2 wt.%), which cannot provide sufficient bond strength between the particles during the granulation process. Less fragmentation and a smaller amount of remnant powders without granulation were observed in the granules prepared with 3 wt.% or more PVA, compared to the case of the samples containing 2 wt.% and 2.5 wt.% PVA. It is believed that the increase in the PVA amount from 2 wt.% to 3 wt.% notably increases the bond strength between the particles. As the binder ratio increased to 3.5 wt.%, the distribution of the grain size improved. It was also observed that some granules were cracked. These cracking defects may have been caused by an increase in the brittleness due to an increase in the amount of PVA. The granulation defects observed in all the samples, such as non-granulated powders and poorly shaped and broken granules have been indicated with red arrows in the SEM images. The high amount of binder in the feed can also lead to some production problems such as clogging of the nozzle and sticking of the slurry to the drying chamber walls. For this reason, the amount of binder in the slurry should not be more than what is necessary. When the highest amount of binder (3.5 wt.% PVA) was applied, nozzle clogging occurred occasionally in the feeding process.
The SEM image analysis results obtained by using Image-J software show that the granule size is proportional to the solid content of the slurry. When the SEM images of the samples with the same PVA content but different proportions of solids (such as Figs. 6(c) and 7(c)) are compared, it is observed that an increase in the granule size, widening of the granule size distribution, a reduction in the amount of non-granular powder, and granular rounding occur if the solid content of the slurry increases from 30 wt.% to 35 wt.%. Besides, when the solid content is increased from 35 wt.% to 40 wt.%, various defects start to form. Such defects include deterioration of the granule morphology, clumping rather than formation of granules, adhering of non-granulated powders to the surface of the already formed granules, and plastic deformation of the granules because of the lack of yield strength. Furthermore, if too high a solid content is considered, the increase in the slurry viscosity might cause various problems during the spray drying process. For example, atomization might not occur as it should, or powders might stick to the spray dryer wall and/or to each other. Such problems not only adversely affect the morphology and properties of the granules but also may cause the drying process to stop or even prevent the device from being used for a while. During the experimental work with the feed containing 40 wt.% solid, an error was encountered that affected the continuity of the spray drying process; it required occasional cleaning of the nozzle. At the end of the process, the morphology and density of the obtained granules were not better than those of the granules obtained from 35 wt.% solid. When the SEM images in Figs. 7(a) to (d) are examined, it is observed that the granules with 40 wt.% solid exhibit partial agglomeration, fracture, disintegration, and deterioration in the surface smoothness. The average size of all the fabricated granules was calculated by using the data obtained from Image-J software. The resultant graph is presented in Fig. 8.
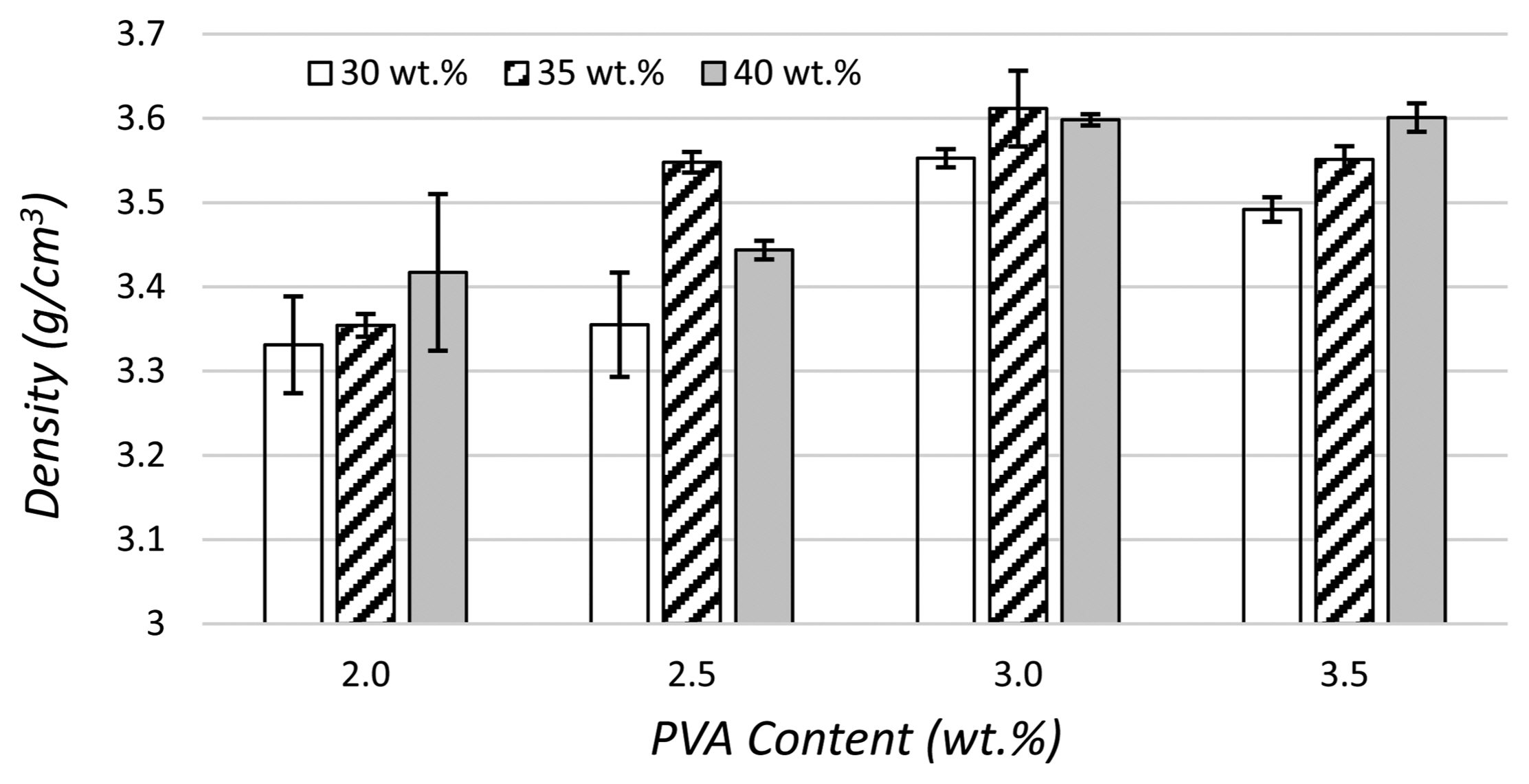
The density values of the produced composite granules and the variations in the density as a function of the amount of PVA in the samples are shown in Fig. 9. The results reveal that the granule density varies in direct proportion to the increase in the amount of PVA in the feed. The highest granule density among all the compositions was obtained for the feed comprising 35 wt.% solids and 3 wt.% PVA.
4. Conclusions
The binder content of the feed slurry is the dominant factor that determines granule morphology. By increasing the amount of PVA in the composition from 2 wt.% to 3 wt.%, the number of broken granules and non-granulated powders decreased. Additionally, the granule morphology became more spherical, the average granule size increased, and the size distribution widened. However, cracking defects developed upon further increasing the PVA amount to 3.5 wt.%.
The solid content of the feedstock is also an important parameter that affects the granule morphology, but it is not as effective as the binder amount. It is observed that increasing the solid content from 30 wt.% to 35 wt.% helped reduce granule defects. However, like the case of the amount of PVA, further increase in the solid content of the slurry (from 35 wt.% to 40 wt.%) results in granule defects that start to form, though the defect formation is not as dramatic as in the case of high PVA feeds.
It should be noted that, to tailor the granule morphology, size, and size distribution, further studies are necessary to design feedstock slurries with optimum solid/liquid ratio and binder amount for obtaining powders with different physical properties. In this study, the best combination of these factors was found to be 35 wt.% solid and 3 wt.% PVA, which resulted in the fabrication of defect-free spherical TiO2 ceramic powders.