1. Introduction
Carbides, borides, and nitrides have exceptional thermomechanical and thermochemical properties and a high melting temperature, and thus, they are often called ultra-high-temperature ceramics (UHTCs). As UHTCs are usually hard-to-sinter materials, sintering additives are needed to densify them, which inevitably degrades their high-temperature properties. Spark plasma sintering (SPS) and hot-isostatic pressing can be used to fabricate UHTCs with fewer sintering additives,1) and chemical vapor deposition (CVD) can help fabricate pure UHTCs with excellent high-temperature performance.2) However, it is difficult to fabricate large-scale materials or materials with a complicated shape by using these techniques.
As an alternative method, melt-solidification of eutectic composites has been receiving considerable attention.3) UHTCs are hard-to-melt materials with extremely high melting temperatures; however, the melting temperatures of UHTCs can be significantly lowered by carrying out thermodynamic reactions, typically eutectic reactions. While many carbides, borides, and nitrides (non-oxides) of UHTCs, such as SiC and TiN, cannot melt at normal pressure by sublimation or decomposition at high temperatures, the eutectic reaction can readily melt and solidify these ceramics to produce composites.
UHTC eutectic composites have a unique self-aligned microstructure, resulting in outstanding mechanical and thermal properties. Directionally solidified eutectics (DSEs) of UHTCs have more sophisticated aligned textures, which suggests that they are promising high-temperature structural materials. Thus far, the use of UHTC eutectic composites has been limited to small-scale materials because such high temperatures were rarely achievable in the past; however, recent advanced technology using lasers or electron beams readily enables the realization of extreme high temperatures, providing practical applications for UHTCs.
Melt-solidification is a short process compared with conventional sintering, and therefore, material research using this process can be conducted efficiently. We investigated the development of UHTC composites using arc-melting, floating-zone melting, and laser melting. Arc-melting makes it easy to search for candidate materials, and floating zone melting can be used to form sophisticated directionally solidified materials. Laser melting can be used to fabricate a large-area solidified material. This paper reviews our research on UHTC eutectic composites and state-of-art laser melting techniques.
2. The Eutectic Reaction
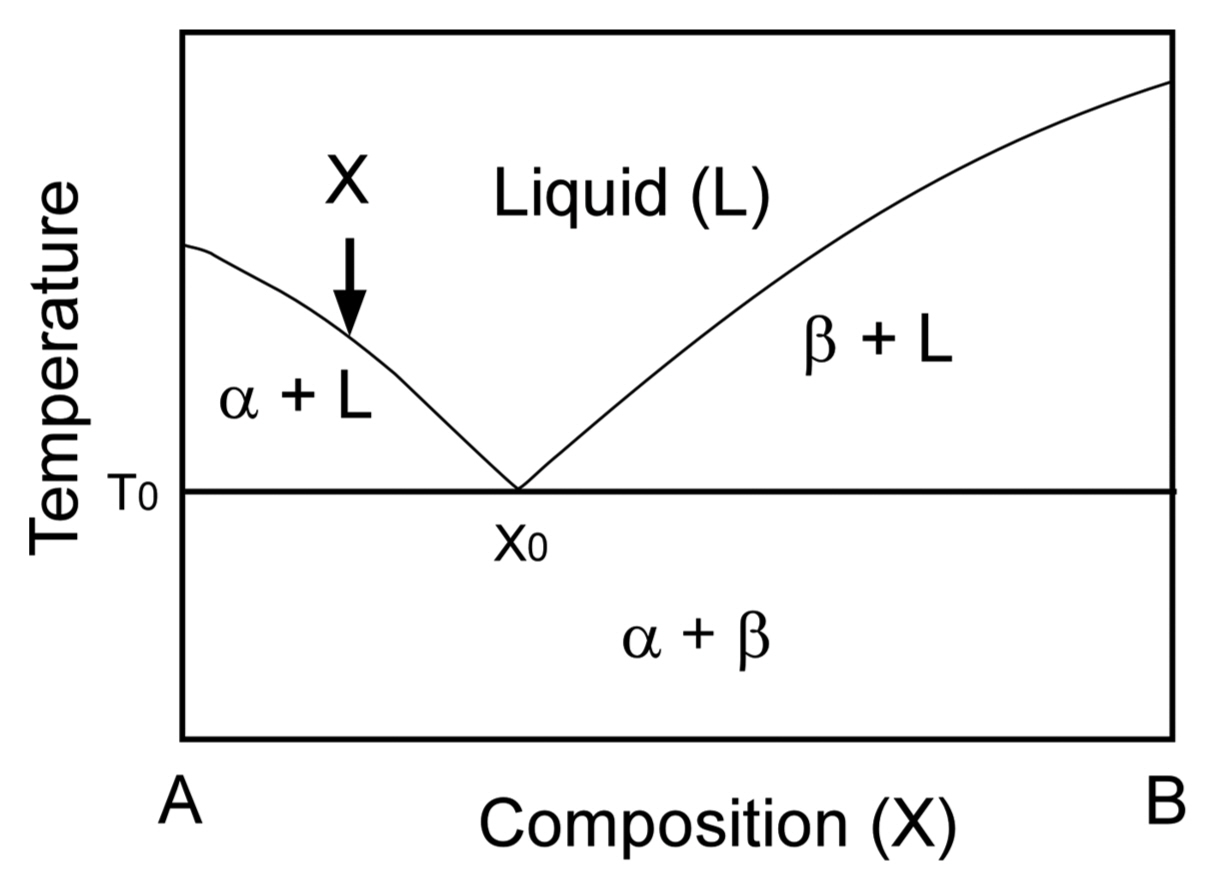
The eutectic reaction is a thermodynamic feature of melt-solidification that results in two (binary) or three (ternary) solids from a multi-component liquid. Fig. 1 presents a typical phase diagram of a binary eutectic system. The melting temperature of A decreases with increasing content of B in a liquid, while that of B also decreases with increasing content of A. At T0, a liquid (L) and two solids, α and β, are in equilibrium. The lowest melting temperature is the eutectic temperature. When a liquid with a composition of X is cooled, the liquid solidifies into α and the composition of X in the liquid changes such that the liquid becomes B-rich close to X0. At T0 (eutectic temperature), the solidification is completed. The first solidified α is a primary phase, and the A-rich region is hypoeutectic. At a composition of X0, α and β simultaneously solidify from the liquid. X0 is the eutectic composition. At the B-rich region, β is the primary phase, and its region is hypereutectic.4) Fig. 2 demonstrates the microstructure of the TiC-TiB2 binary eutectic system.5) At a TiC-rich composition (hypoeutectic), TiC first solidifies as a primary phase, and finally, the TiC-TiB2 binary eutectic (BE) solidifies (Fig. 2(a)). At a TiB2-rich composition (hypereutectic), TiB2 solidifies as a primary phase, and the binary eutectic (BE) finally solidifies (Fig. 2(b)). The eutectic composition with a uniform texture can be easily recognized from microstructural observations.
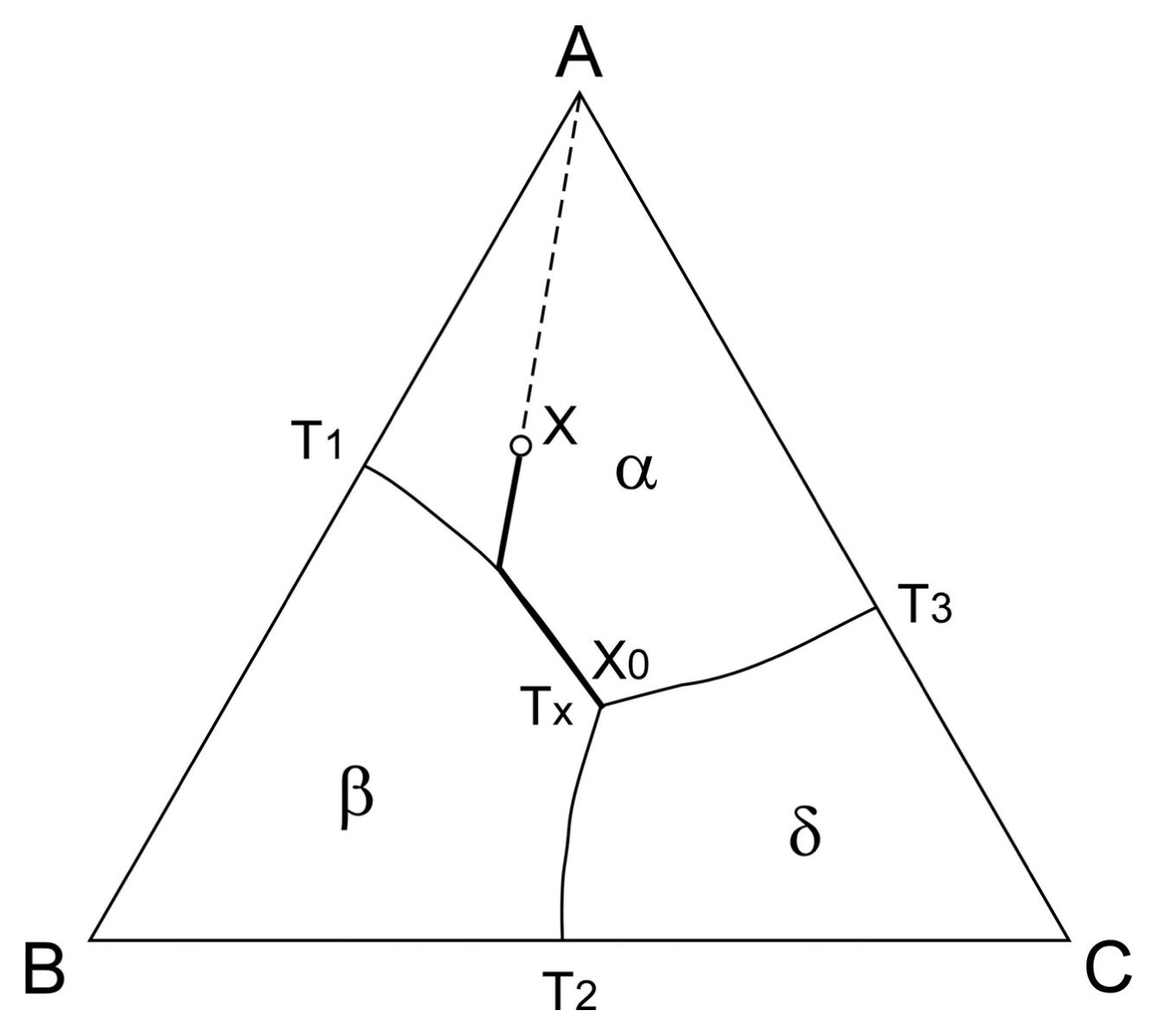
A ternary eutectic phase diagram is shown in Fig. 3, where the A-B, B-C, and A-C systems are eutectic with eutectic temperatures of T1, T2 and T3, respectively. α, β, and δ are primary phases of A-rich, B-rich and C-rich regions, respectively. Tx is a ternary eutectic temperature. When a liquid with the composition of A cools, at first, the primary phase of α solidifies, and then the composition of the liquid changes along the T1-Tx binary eutectic line. Finally, a ternary eutectic of the composition of X0 solidifies. Therefore, by changing the composition of the liquid, one can fabricate various kinds of composites in which a coarse primary phase is dispersed in a eutectic matrix composite or fine binary eutectics are mixed with a ternary eutectic. Fig. 4 depicts the microstructure of the B4C-TiB2-SiC ternary eutectic system.6) The microstructure is a mixture of the SiC-B4C binary eutectic (BE) and the B4C-TiB2-SiC ternary eutectic (TE). As the ternary eutectic solidifies later than the binary eutectic with a slower cooling rate, the texture of the ternary eutectic becomes coarser than that of the binary eutectic. By utilizing the eutectic reaction, not only structural ceramics but also various functional ceramics can be developed.7)
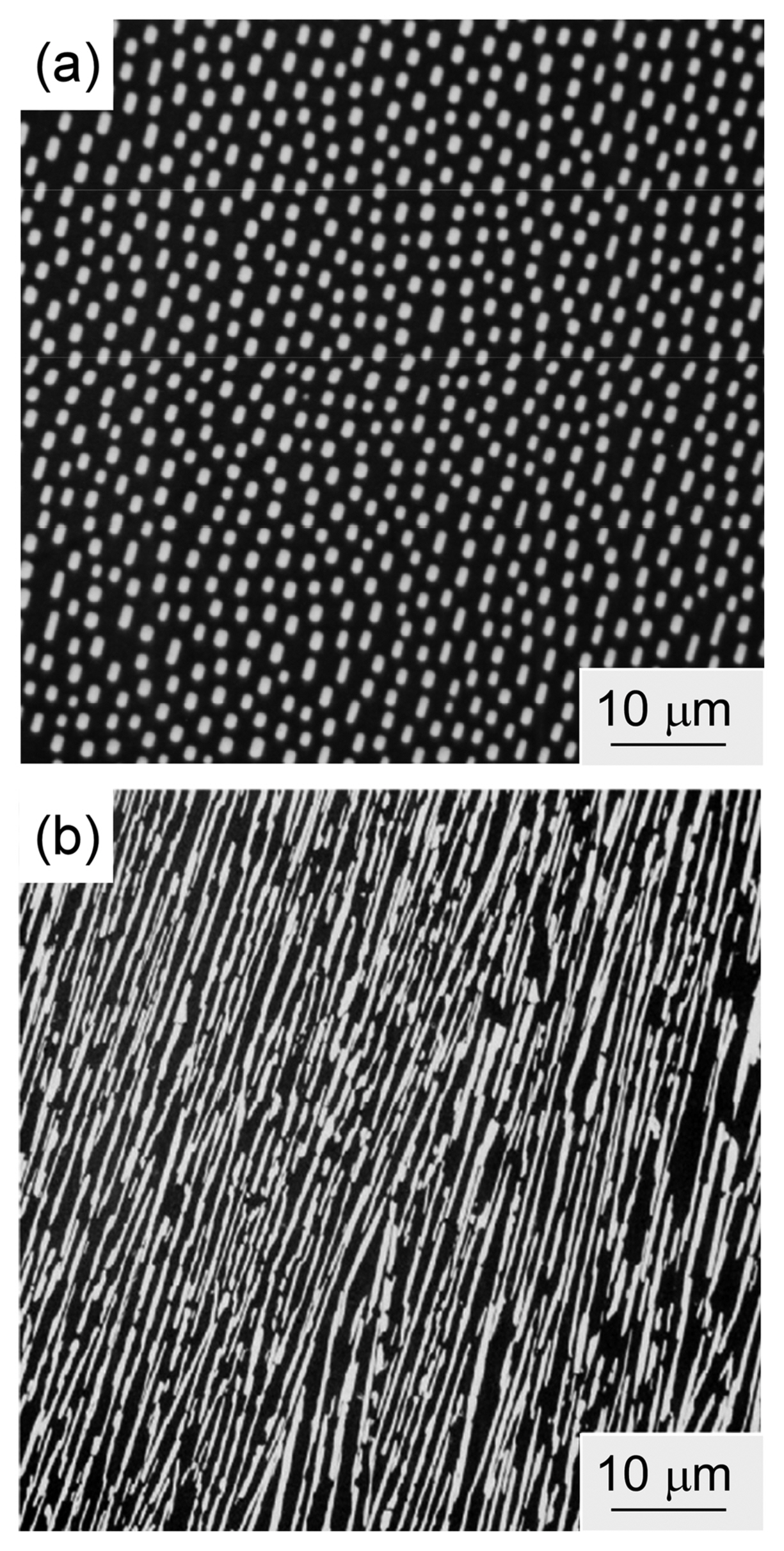
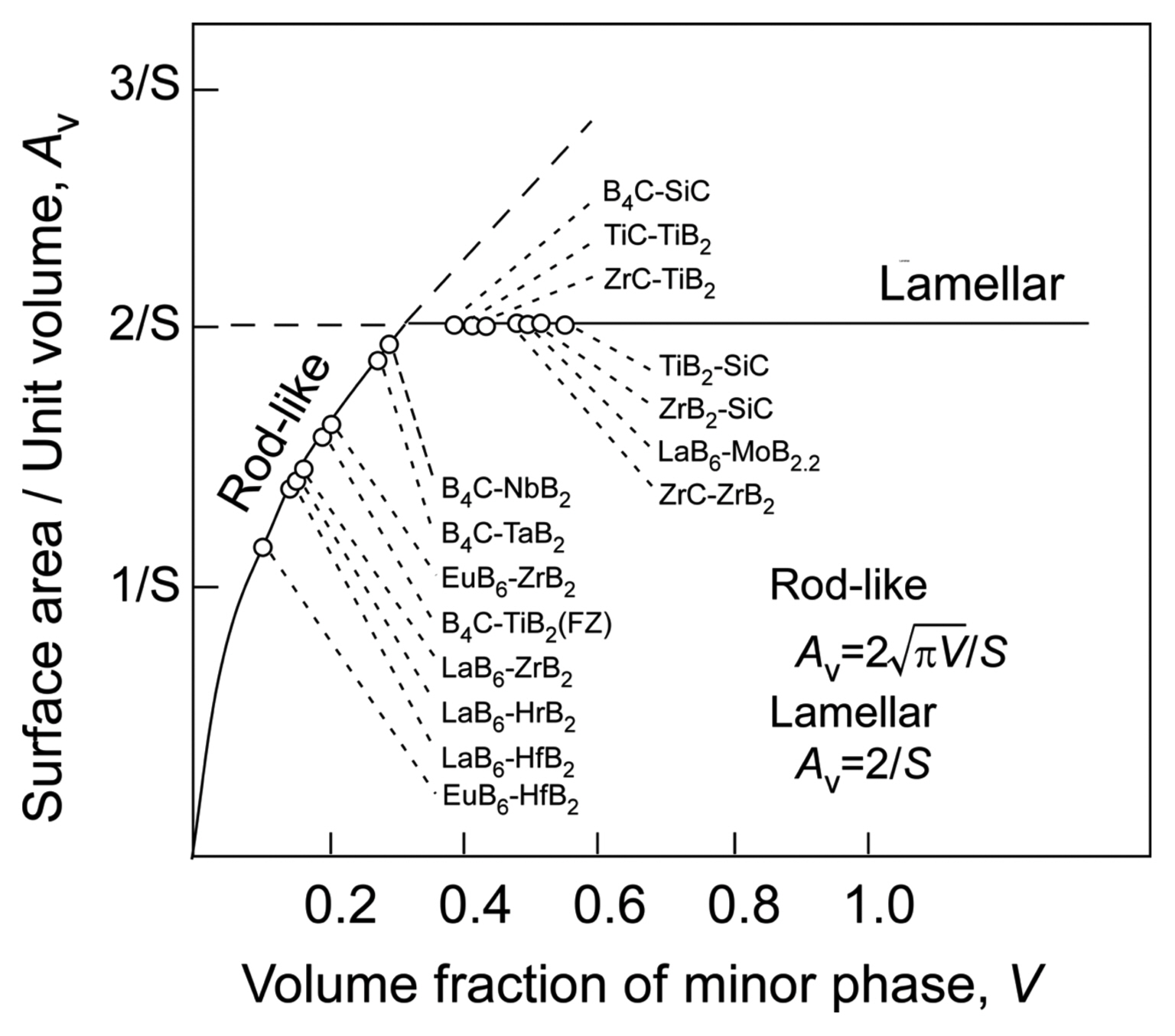
The microstructure of eutectic composites is typically either rod-like (fibrous) or a lamellar texture; however, sometimes, an intermediate labyrinth (or Chinese character-like) texture can be formed. The microstructure basically changes with the composition of the material; the rod-like texture forms when the minor-phase volume fraction of a material is less than ~ 30 vol%. Fig. 5(a) shows the microstructure of the B4C-TiB2 eutectic (B4C-26 vol%TiB2): rod-like TiB2 (white) in a B4C matrix (black).8) Fig. 5(b) shows the microstructure of the lamellar B4C-SiC eutectic (B4C-49 vol%SiC): lamellar SiC (white) with lamellar B4C (black).9) The microstructure of eutectics is mainly determined by minimizing the interface area of the minor phase. A rod-like texture is theoretically expected for a minor-phase volume fraction of less than 1/π (31.8 vol%).10) Fig. 6 summarizes the relationship between the surface area/unit volume (Av) and the volume fraction of the minor phase (V) for various eutectic composites. In almost all systems, a rod-like texture is formed at a minor-phase volume fraction of less than ~ 30 vol%. A lower surface energy would result in a lamellar texture at a volume fraction of less than ~ 30 vol%. If there is some strong interface interaction, a rod-like or labyrinth texture can be observed when the minor-phase volume fraction is more than ~ 30 vol%. The Av is given by
2 π V / S
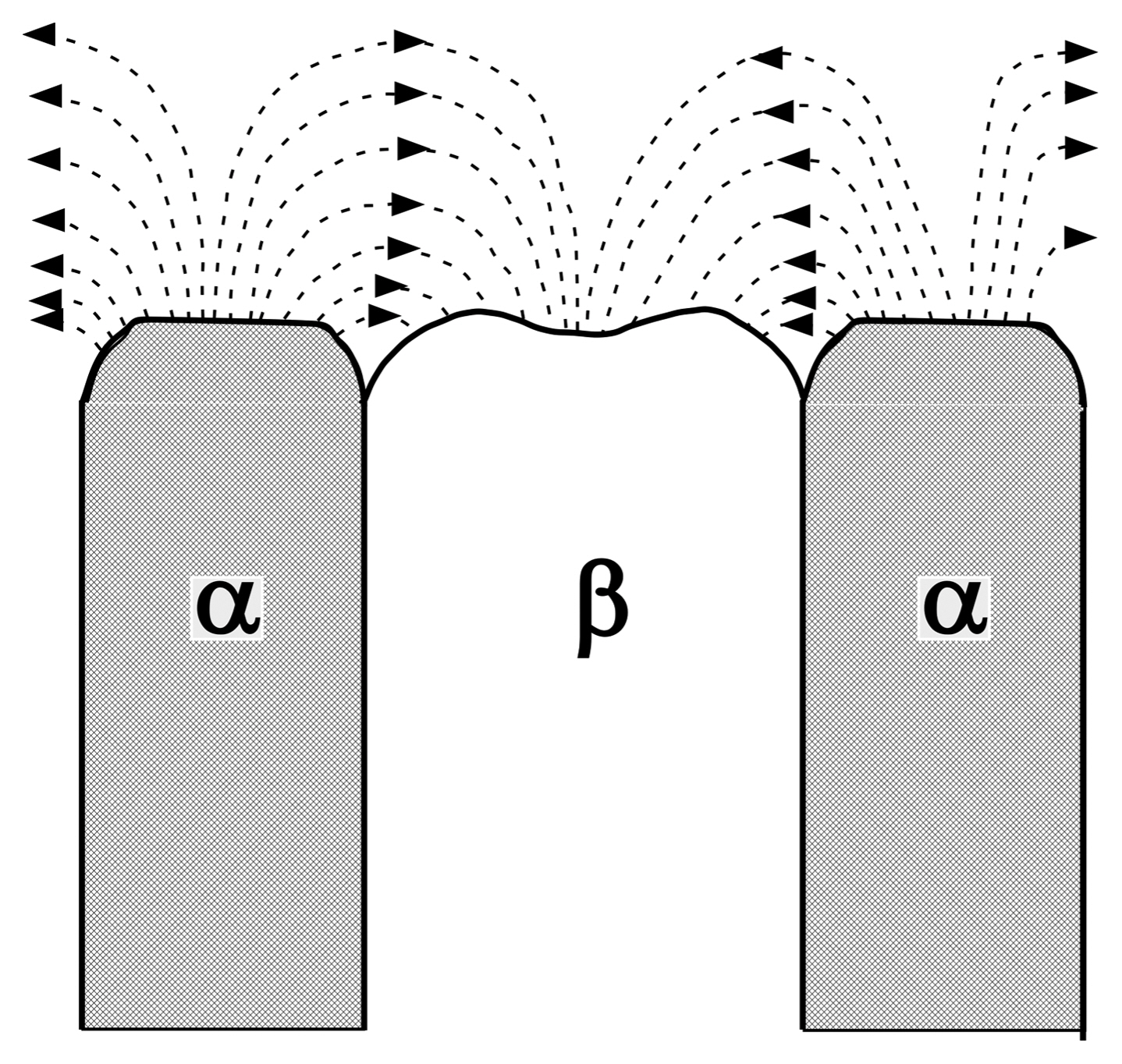
Figure 7 depicts a schematic diagram of the liquid/solid interface of the binary eutectic. At the solid-liquid interface, when the α phase solidifies from a liquid phase, the B component should diffuse into the neighboring β phase. Therefore, when the cooling rate is fast, the diffusion length becomes shorter, and the texture becomes finer with an increasing cooling rate. The diffusion of A and B in a liquid significantly affects the inter-lamellar (or inter-rod) spacing in the eutectic system, obeying Eq. (1):11)
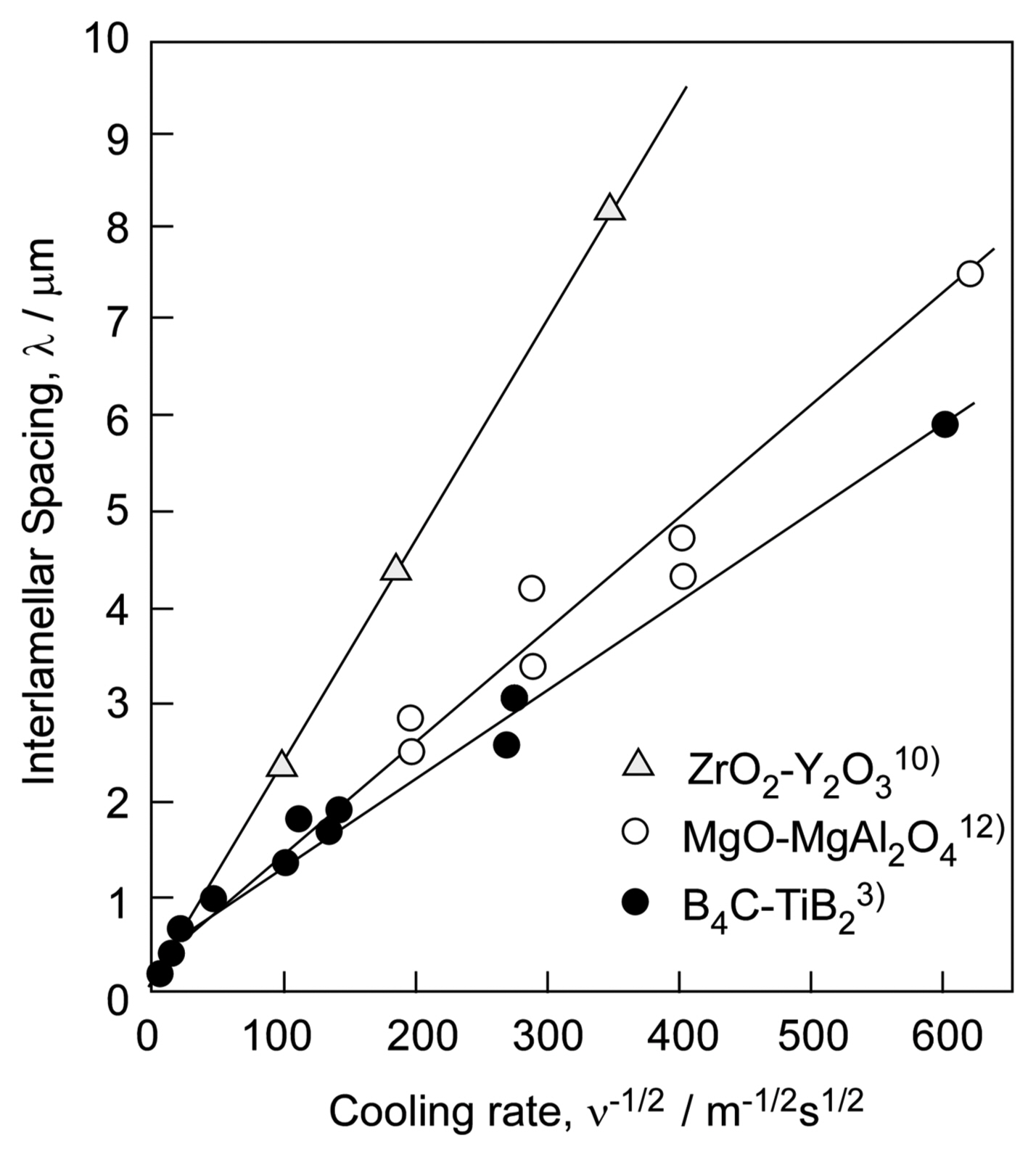
where ν is the cooling (solidification) rate, and λ is an interlamellar (or inter-rod) spacing. K is a constant. Fig. 8 presents the relationship between the inter-lamellar (inter-rod) spacing and cooling rate for the B4C-TiB2,3) ZrO2-Y2O310) and MgO-MgAl2O4 eutectic composites.12) The ν vs. λ−1/2 relationship is linear. Most eutectic systems, irrespective of whether they are metals, oxides, and non-oxides, obey Eq. (1). By using laser melting, significantly high cooling rates can be used to fabricate a material with a fine texture, while the use of a small cooling rate by floating zone melting results in a material with a coarse eutectic texture. By changing the cooling rate, the microstructure of eutectic composites can be controlled.
In general, the performance of a material depends on the size of its grains: the smaller the grain size is, the higher the hardness and strength are; this is known as the Hall-Petch relationship.13,14) The grain sizes of ceramics produced by sintering are usually not smaller than that of the source powder. However, in eutectic composites, the grain size (inter-lamellar spacing) decreases with the square root of the cooling rate. The inter-lamellar spacing decreases in the order of floating zone melting, arc melting, and laser melting, and a finer texture and stronger ceramic can be developed by using a process with a higher cooling rate.
3. Binary and Ternary UHTC Eutectic Composites
Al2O3-GaAlO3 oxide eutectic composite ceramics have been studied as structural ceramics owing to their high strength and high ductility at high temperatures.15) The DSE of Al2O3-GaAlO3 is also promising as a functional ceramic for scintillating.16) While oxide eutectic composite ceramics have been intensively investigated, studies on UHTC eutectic composites is rather scarce. Most studied UHTCs are SiC-based composites. As SiC has high strength, high thermal shock, and oxidation resistance at high temperatures, it has been widely used as a structural ceramic.17) In order to improve the properties of SiC, many SiC-based composites have been developed by solid-state sintering. As SiC sublimates, it does not melt at a normal pressure at high temperatures. However, SiC can melt by adding a second phase, such as B4C, by the eutectic reaction. The earliest studied UHTC eutectic composite was the SiC-B4C system.18) The SiC-B4C eutectic composite was also reported to have high thermoelectric performance.19) The DSE of SiC-B4C showed excellent mechanical and thermochemical properties.9) Although SiC would severely degrade by active oxidation at a low oxygen partial pressure and high temperatures, such as in a space,20) studies have been widely conducted to improve the oxidation resistance of SiC-ZrB2.21) SiC-ZrB2 oxidizes to SiO2 and B2O3, whereby B2O3 would suppress the active oxidation and could heal the cracks on the surface of SiO2. The SiC-ZrB2 system is binary eutectic. The SiC-ZrB2 eutectic composite has higher oxidation resistance than the SiC-ZrB2 sintered composite.22) Fig. 9 depicts the microstructure of the SiC-ZrB2 eutectic composite. The eutectic composition is SiC-51 vol%ZrB2.22) Although the second phase (ZrB2) is more than 30 vol% where the lamellar texture would be expected, the microstructure is a labyrinth texture, a partially connected rod-like texture. Hexagonal ZrB2 has a strong interface connection with the cubic SiC matrix, resulting in the labyrinth texture.
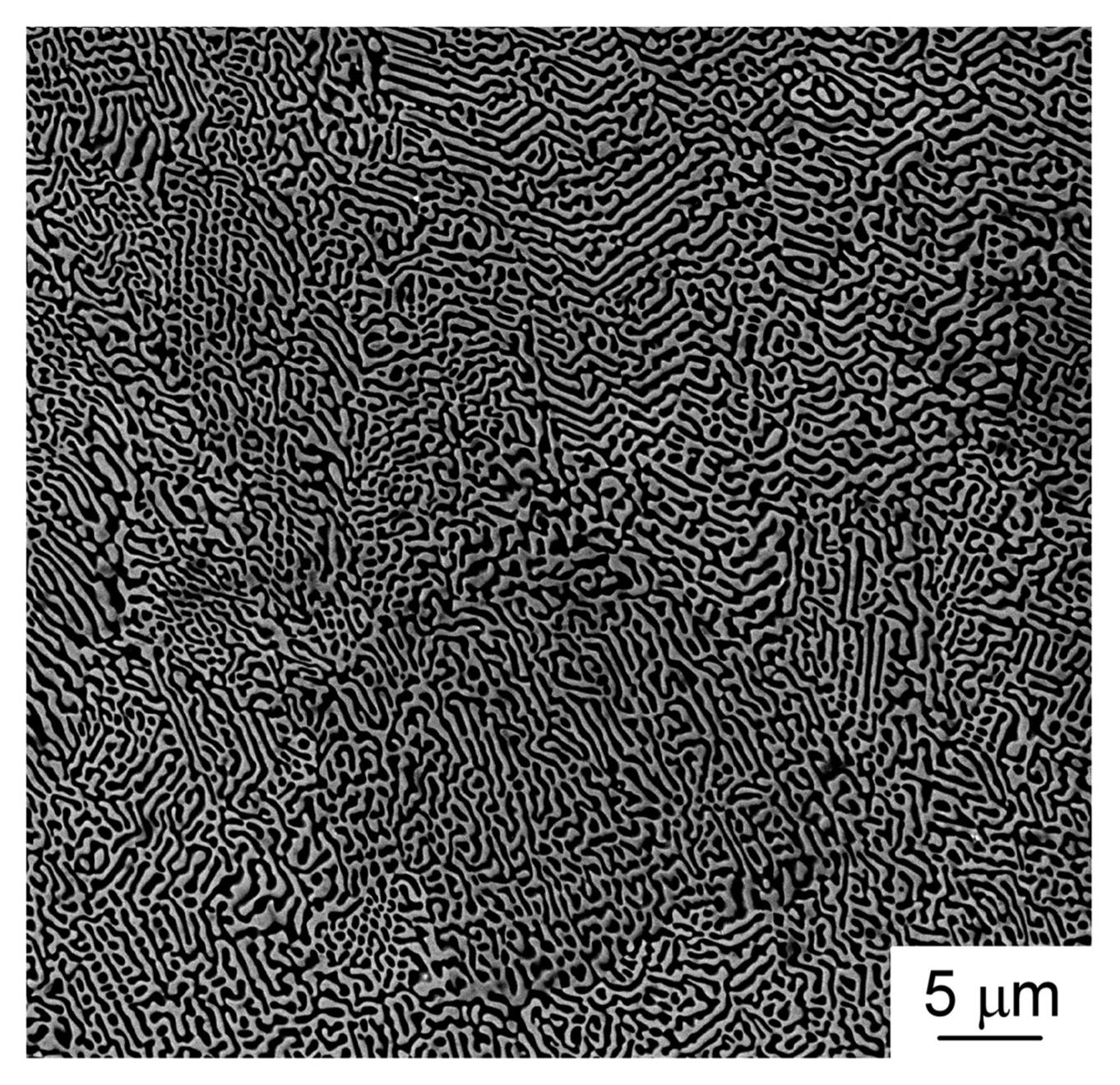
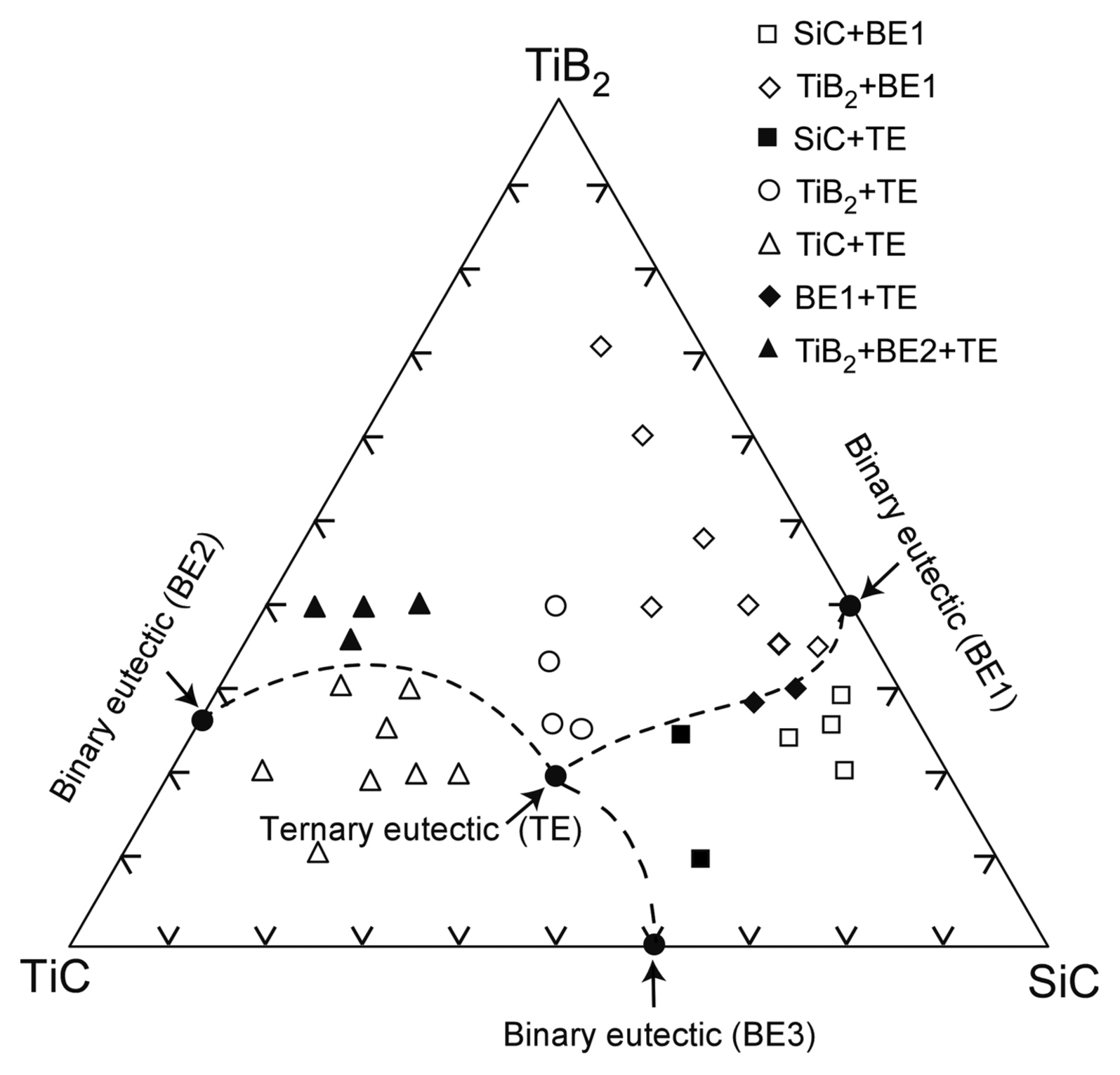
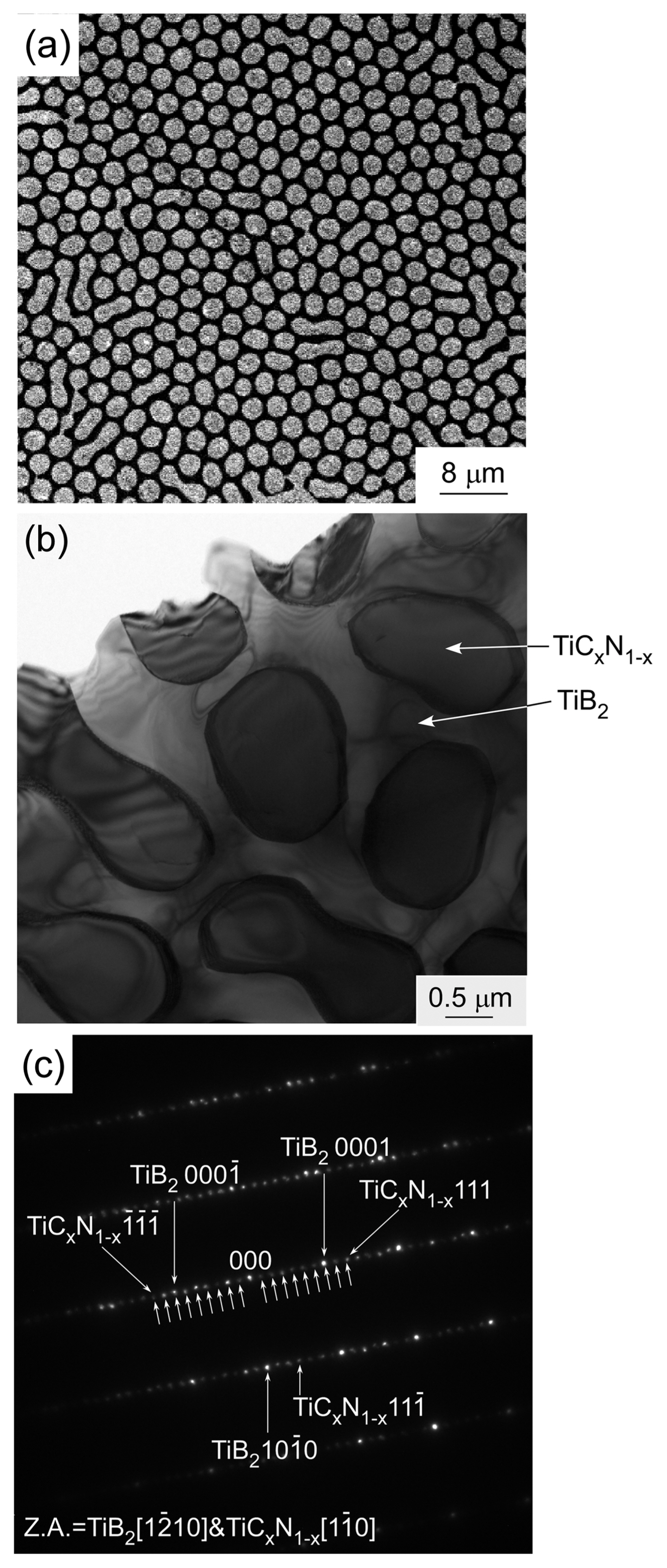
Figure 10 depicts the SiC-TiB2-TiC ternary phase diagram. Each end member is a binary eutectic, and therefore, the SiC-TiB2-TiC system is ternary eutectic.23) TiB2 has an AlB2 structure like that of ZrB2. SiC-TiB2 is a eutectic whose eutectic composition is SiC-45 vol%TiB2.24) TiB2 has high electrical and thermal conductivities, and thus, the SiC-TiB2 eutectic composite has high strength and high thermal stability with high electrical and thermal conductivities. The hardness of the SiC-TiB2 eutectic composite is the highest owing to its fine microstructure.24) The SiC-TiB2 eutectic composite also has a labyrinth texture. The SiC-TiC system is eutectic, and its eutectic composition is SiC-60 vol%TiC. SiC and TiC have a cubic structure, with no specific relationship between the two phases; the SiC-TiC eutectic composite has a common lamellar texture. The SiC-TiC composite has been widely studied. Sintered SiC-TiC composite exhibits high densification and strength when its composition is close to the eutectic composition.25) SiC-TiC composites have been also fabricated through CVD26) and sputtering.27) The vapor-deposited SiC-TiC composites have a fine microstructure, and the SiC-TiC composite formed using CVD has a high fracture toughness, which is similar to that observed for the SiC-TiC composite with the eutectic composition. Vapor-phase deposited materials with a eutectic composition can also have significantly fine microstructures. The TiC-TiB2 system is a binary eutectic whose eutectic composition is TiC-32 vol%TiB2.5) As the volume fraction of the second phase (TiB2) is close to 30 vol%, the microstructure of the TiC-TiB2 eutectic composites is a mixture of rod-like and lamellar texture. TiC has a wide-ranging stoichiometry from TiC0.5 to TiC1.0, and the eutectic temperature decreases with decreasing carbon content in TiC. Both TiC and TiN have a rock-salt structure and exhibit similar properties. The TiB2-TiN system is also eutectic. The TiC-TiN system is a completely solid-solution, and therefore, the TiB2-TiCN system is eutectic, and its eutectic composition is TiB2-69 vol% TiCxN1−x at x = 0.67.28) Fig. 11 shows the surface microstructure of the TiB2-TiCxN1−x eutectic composite. A huge numbers of rod-like TiCxN1−x phases can be seen in the TiB2 matrix (Fig. 11(a)). Some TiCxN1−x rods have a hexagonal shape, and two or three TiCxN1−x rods are partially connected. It is unusual for the TiCxN1−x phase still to be rod-like, even at ~ 70 vol% of TiCxN1−x. <0001>TiB2 is parallel to <111>TiCxN1−x, and (1120) TiB2 is parallel to (202)TiCxN1−x. Both TiB2 and TiCxN1−x have a single crystalline structure. TiCxN1−x can be coherently connected to TiB2. A strong interaction at the TiCxN1−x/TiB2 interface may result in a rod-like texture at a high TiCxN1−x volume content of ~ 70 vol%. A dark contrast can be seen at the interface between TiB2 (white) and TiCxN1−x (gray) (Fig. 11(b)). The diffraction of the gray contrast area implies a long-ranged structure, where nine spots of (111) TiCxN1−x can be seen between (12̄10)TiB2 at x = 0.67 (Fig. 11(c)). Nine layers of d111 (2.25 nm) agree with seven layers of d12̄10 (2.24 ~ 2.25 nm). Even in the simple system of TiB2-TiCxN1−x, a new compound or a new structure can be found.29) Research on the use of UHTCs at temperatures higher than their melting temperature is scarce. More new compounds or new structures may be found by using melt-solidification. As Zr has similar chemical properties to Ti, like Ti compounds, the ZrB2-ZrC,30) ZrB2-ZrN,31) and ZrB2-ZrCN32) systems are also binary eutectic, where the eutectic composition of the second phase (ZrC, ZrN, and ZrCN) is ~ 60 vol% with a rod-like texture. The ternary eutectic composition of SiC-TiB2-TiC is 42SiC-26TiB2-32TiC (vol%). The B4C-TiB2-SiC system is also ternary eutectic, and the eutectic composition is 64B4C-7TiB2-29SiC (vol%).6) Fig. 12 depicts the microstructure of directionally solidified ternary SiC-TiB2-TiC and B4C-TiB2-SiC eutectic composites. Both ternary eutectic composites have a lamellar texture. The SiC (black) and TiC (white) phases are parallel, but the TiB2 (gray) phase is slanted toward the SiC and TiC phases in the SiC-TiB2-TiC eutectic composite (Fig. 12(a)). The B4C, TiB2, and SiC phases are in parallel with a lamellar texture. The TiB2 phase disperses finely at the B4C/SiC interface (Fig. 12(b)).
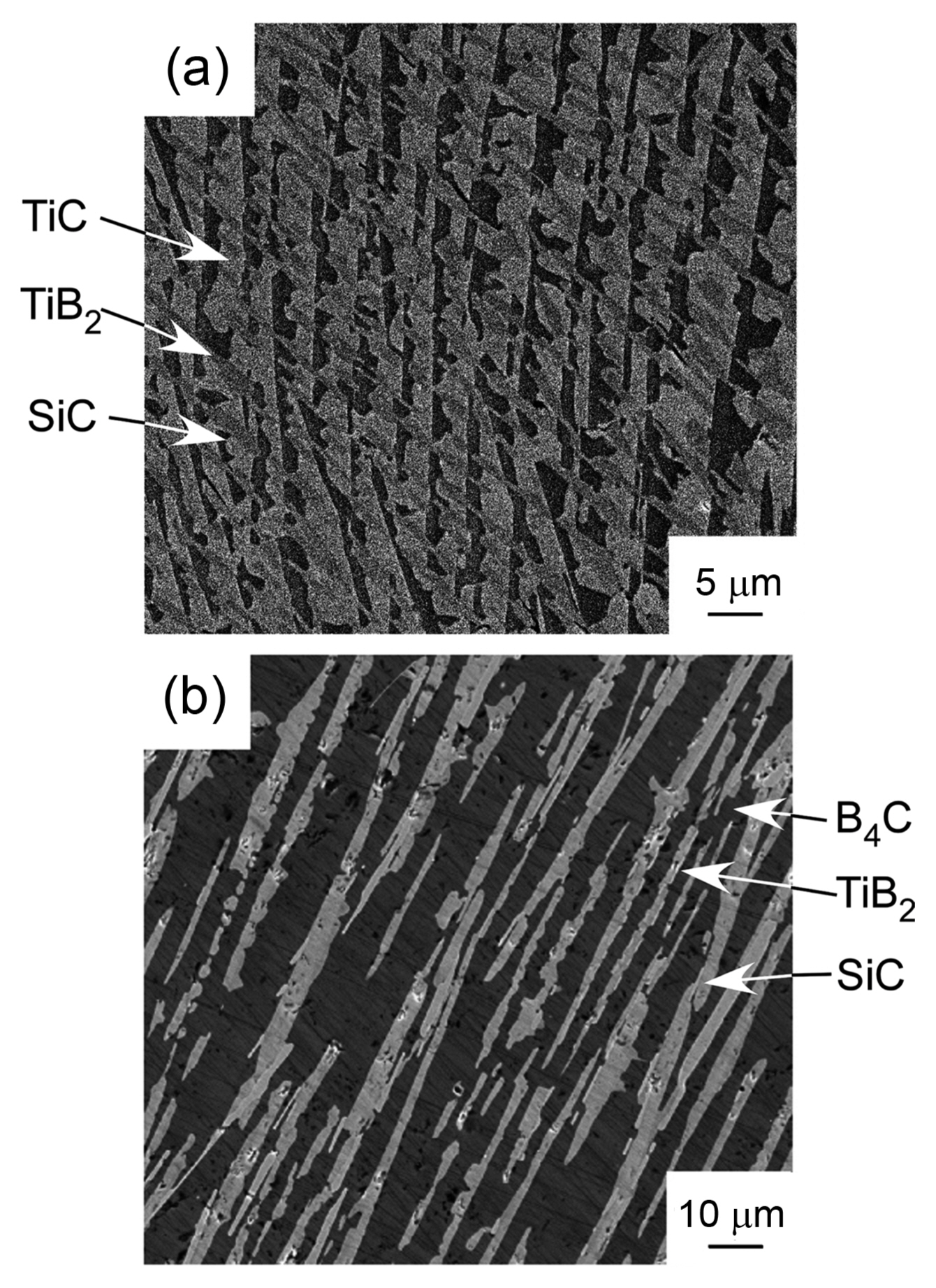
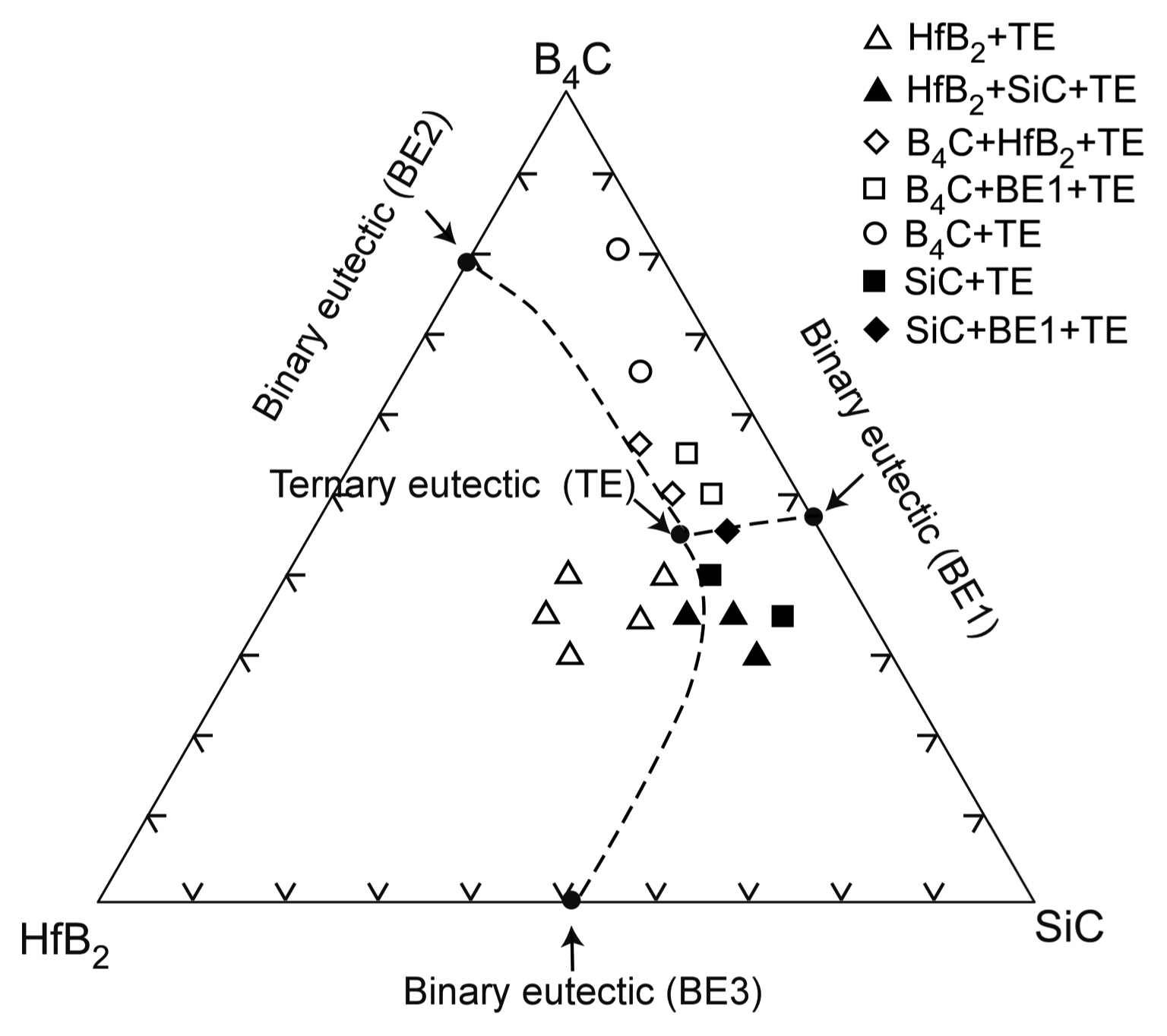
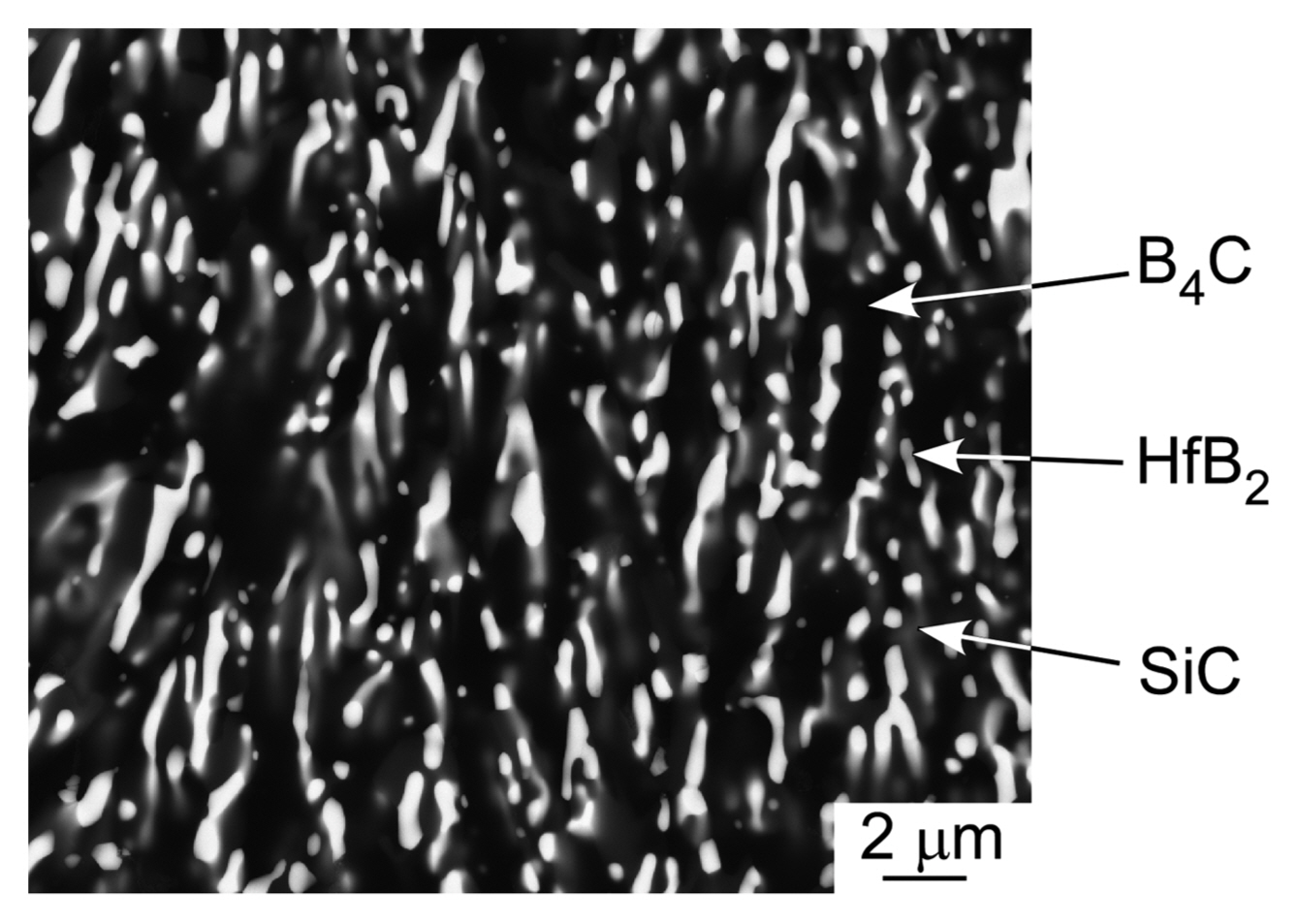
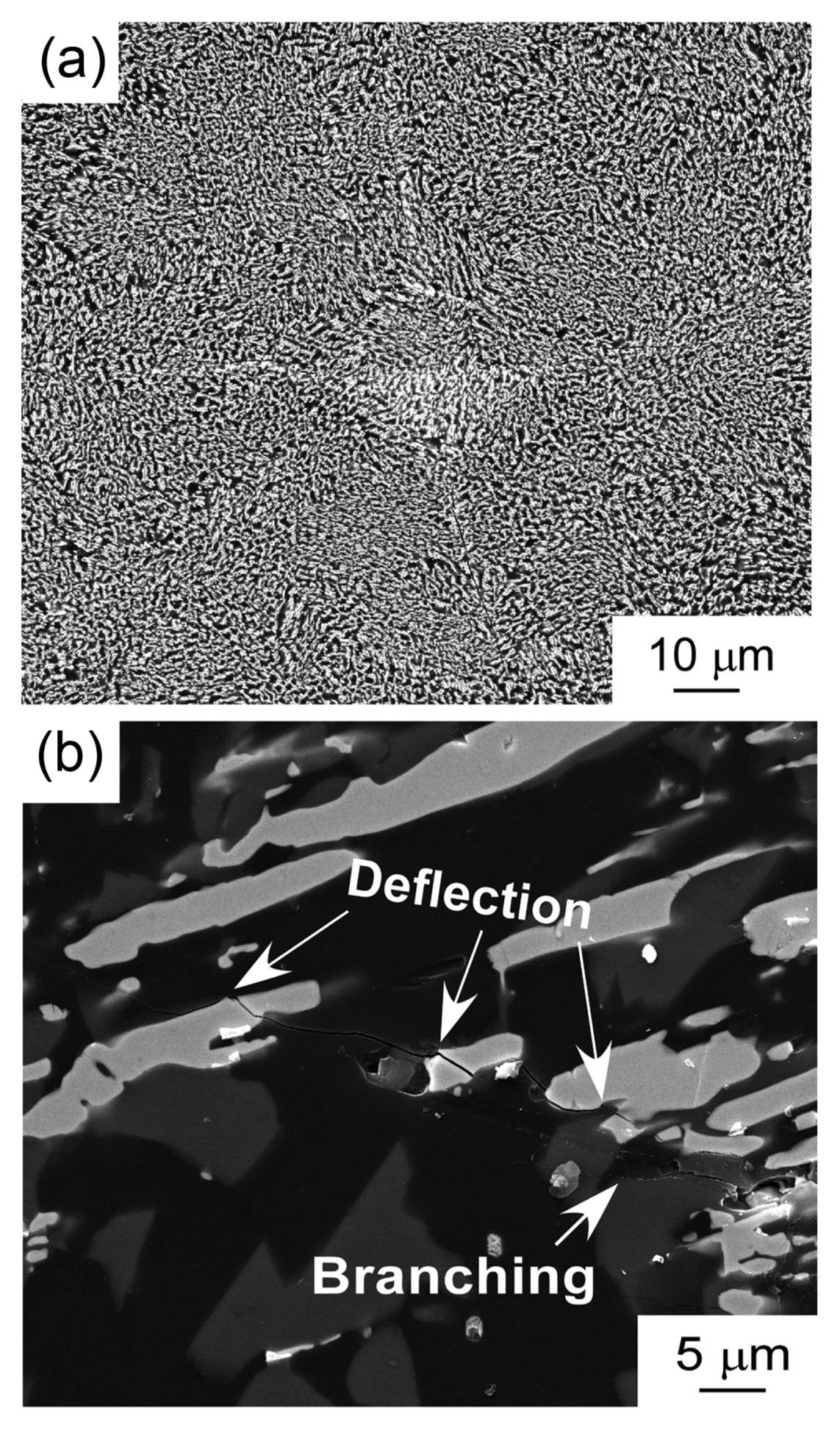
Hf has similar chemical properties as Zr and Ti. Therefore, the phase diagram of Hf-based compounds is also similar to those of Zr- and Ti-based compounds. Fig. 13 shows the ternary phase diagram of the B4C-HfB2-SiC system.33) As the melting temperature of Hf (2222°C) is higher than those of Ti (1812°C) and Zr (1852°C), Hf-based compounds have higher melting temperatures then Ti- and Zr-based compounds. Many Hf-based compounds are promising UHTCs. The B4C-SiC,7) B4C-HfB2,33) and SiC-HfB2 systems are binary eutectic, and the ternary eutectic composition is 49B4C-14HfB2-37SiC (vol%). Fig. 14 depicts the microstructure of the B4C-HfB2-SiC ternary eutectic composite by arc melting, where B4C is black, SiC is gray, and HfB2 is the white phase. The three phases are finely dispersed. As the binary and ternary compounds containing SiC, B4C, and HfB2 are promising UHTCs, the mechanical properties (hardness and fracture toughness, KIC) of these composites have been investigated. Fig. 15 presents the Vickers hardness and fracture toughness (KIC) of binary and ternary B4C-HfB2-SiC composites.34-42) Generally, high hardness materials have a low fracture toughness. The hardness has a trade-off relationship with fracture toughness. Although many researchers have tried to develop high hardness with high fracture toughness ceramics by using conventional solid-state sintering, great effort is needed to produce good materials by sintering. Instead, melt solidification methods such as arc-melting are easy, and a short time is needed to research high performance ceramic materials. The B4C-HfB2-SiC eutectic composites show higher hardness and toughness than those fabricated by hot-press (HP) and SPS. Fig. 16 depicts the Vickers indentation and crack formation of the B4C-HfB2-SiC eutectic composite. As the microstructure is small, the cracks are deflected and branched, causing high fracture toughness.
4. DSE Composite by Laser Melting
Although floating zone melting has often been used to fabricate DSEs, the size of UHTCs is usually limited to several tens of millimeters. On the other hand, a high temperature technology using a laser or electron beam is currently under development, and a high temperature of more than 3000°C can be readily produced. In particular, laser technology does not require high vacuum, so it is appropriate to melt UHTCs, particularly those containing volatile compounds such as SiC and TiN. Laser technology has been widely used to melt metals for cladding and welding; however, it has rarely been used to melt ceramics. While the direction of solidification in floating zone melting is axial to the materials, it is inclined to be in the scan direction of the laser. There is basically no limitation on the size of materials in laser melting as long as the laser can scan them. Larrea et al. melted and solidified the ZrO2-Al2O3 eutectic composite by using a CO2 laser (1 kW/cm2).43) The laser was modified to have a length and width of 20 and 0.5 mm, respectively, by using a parabolic mirror, and the ZrO2-Al2O3 eutectic composite was directionally melted and solidified using a scanning laser. Chen et al. used the Nd:YAG laser (2 kW) with a length and width of 12.7 and 5 mm, respectively, and the WC-W2C eutectoid-like composite was fabricated.44)
Arc-melting is a type of pseudo-directional solidification, in which melted materials are solidified directionally from a water-cooled copper hearth. Arc-melting allows for the easy melting of numerous metals, and it is a useful method for searching for high-performance materials in a short time. On the other hand, laser melting has no size limitation, and its solidification rate is high and directional when a scanning laser is used. Once a good material is found by arc-melting, laser melting can be applied for practical applications to fabricate wide-area UHTC eutectic composites. SiC is a promising UHTC, and CrB2 has high corrosion/oxidation resistance with a relatively low melting temperature (2100°C).45) This is advantageous for the fabrication of wide-area ceramic materials by laser melting. We first melted and solidified SiC-CrB2 composites by arc-melting to determine their eutectic composition; then, laser melting was applied to obtain the SiC-CrB2 eutectic composite. The SiC-CrB2 system is a binary eutectic whose eutectic composition is SiC-81 vol%CrB2, and it has a rod-like texture. As shown in Fig. 8, the microstructure of the eutectic composite becomes finer as the cooling rate increases. In the case of laser melting, the cooling rate increases with an increasing scanning rate.
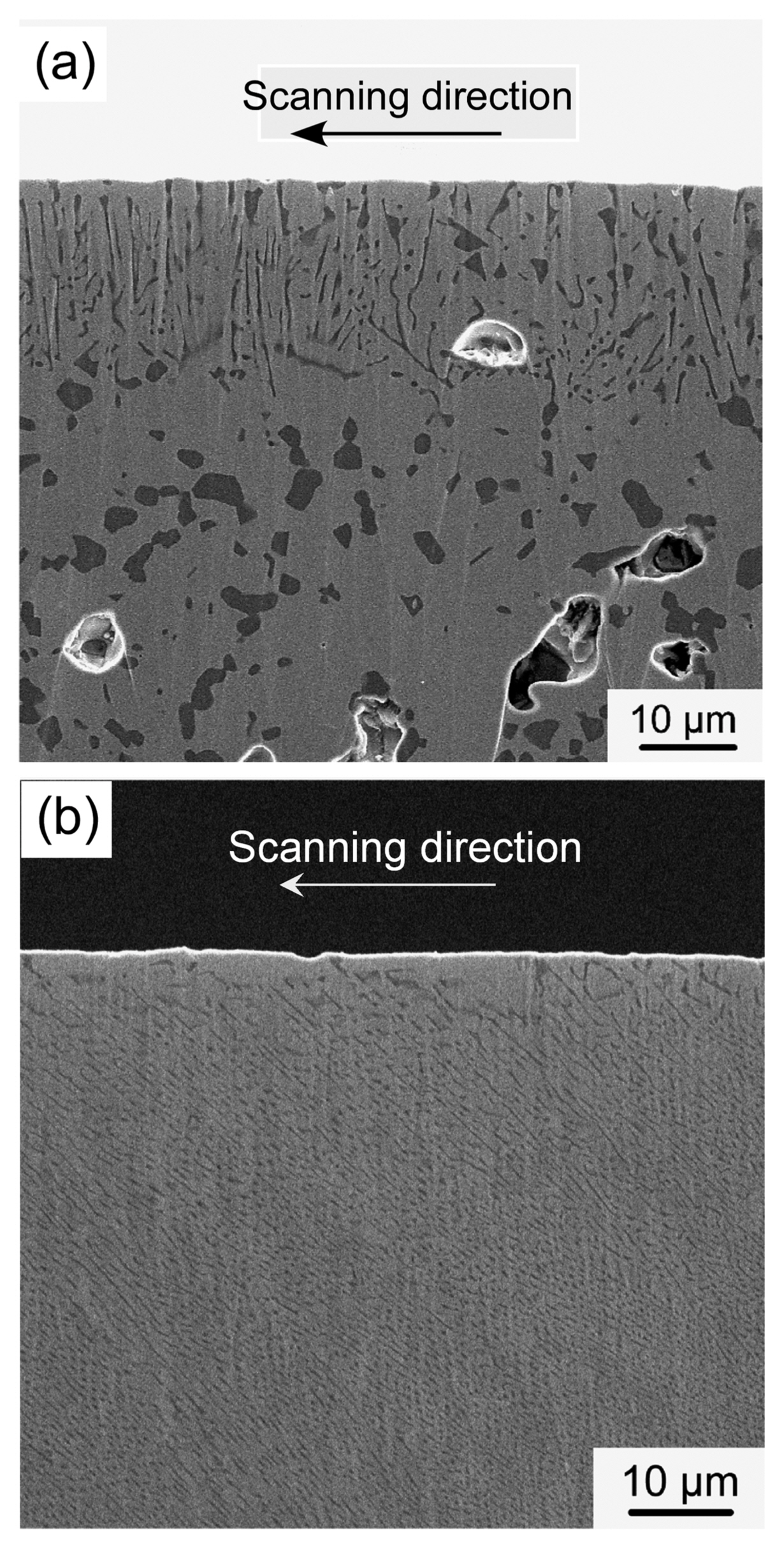
Figure 17 presents the effect of the laser scanning rate on the cross-sectional microstructure of the SiC-CrB2 eutectic composite. The composite has a course texture and a melted depth of about 20 μm at a scanning rate of 0.3 mm/s (Fig. 17(a)). SiC grains (black) are coarsened, and the depth of the melted area is thin. A melted pool forms on the surface when the scanning rate is slow. The laser is reflected on the liquid pool surface. Immediately below the melted zone, the SiC-CrB2 composite is solid-state sintered. The laser can also be used for sintering. At the laser scanning rate of 1.8 mm/s, the melted depth is about 300 μm with no melted pool; furthermore, the composite has a slightly slanted directionally oriented rod-like texture (Fig. 17(b)). CrB2 has a hexagonal AlB2 structure, and the growth direction is <0001>. As the laser scan has a transverse direction, the <0001> oriented CrB2 is slightly slanted toward the scanning direction of the laser.
5. Summary
Ceramics have generally been fabricated by solid-state sintering because they have a high melting temperature; therefore, the melt-solidification process (casting) has rarely been applied to ceramics, particularly UHTCs. However, by using the eutectic reaction, UHTCs can be melted and solidified. The eutectic reaction enables the fabrication of ceramics with a self-aligned microstructure by controlling the composition and cooling rate. Research on UHTCs above their melting temperatures has rarely been conducted. The phase diagrams of UHTCs in terms of the melting temperature are also not well known. Further study is, therefore, needed. With the advancement of high-temperature technology, such as lasers, the DSE of UHTCs can be readily produced and used for high-temperature applications.