1. Introduction
As one of the growth-limiting nutrients, phosphorus is usually regarded as the most dominant factor accelerating water eutrophication. Excessive amounts of phosphorus have been released into aquatic ecosystems, causing undesirable ecological and economic consequences.1) In recent years, increasing attention has focused on agriculture as a pollution source, particularly on phosphorus transfer.2) The cycle of phosphorus transformations between the various components of the ecosystem has a crucial influence on the water and nutrient balances.3) As one of the forms of bioavailable phosphorus that exists in the environment, condensed phosphates have been less investigated.4) Although some of the condensed phosphates are not directly bioavailable, they can be hydrolyzed to orthophosphates in certain conditions, which serve as potential reservoirs for nonpoint phosphorus sources.5,6) Halliwell et al. reported that the half-life of detergent phosphates (triphosphate) in waste waters was 7.3 h at 15°C and 3.0 h at 20°C, with the most likely decomposition mechanism being enzymatic hydrolysis. 7) Tripolyphosphate (TPP; Na5P3O10, a condensed phosphate) is widely used as an auxiliary washing agent and has the molecular structure -[PO4]-[PO3]-[PO4].8) TPP is also used in the food industry for peptidization, sequestration, cross-linking of starch, and pH adjustment.9) Previous studies have confirmed that TPP is strongly and rapidly adsorbed to aluminum hydroxide, ternary CaMgAl-layered double hydroxides, and MgCaFe-Cl-LDH. Guan et al.8) suggested that the small crystals formed on the surface of aluminum hydroxide contributed more to the fast adsorption than the surface sites. A synergic enhancement was observed for TPP removal over MgCaAl-Cl-LDH,10) since the release of aluminum was in the form of Al(OH)4− and the transport process of TPP on MgFe-Cl-LDH was mainly surface adsorption and near-edge intercalation. Phosphate adsorption at the solid/solution interface of metal (hydr)oxides, especially iron and aluminum (hydr)oxides, plays an important role in the transport and bioavailability of phosphorus.11,12) Goethite (a-FeOOH) was found to be relevant for organic matter adsorption in natural environments.13,14) In addition, natural organic matter (NOM) was also considered to be important for the phosphorus adsorption process.15,16)
In the present research, the adsorption of TPP on goethite under different conditions (pH, electrolyte, time, and temperature) was studied and the effects of NOM (HA, FA) on TPP adsorption were also investigated.
2. Experimental Procedure
2.1. Apparatus and reagents
Measurements of the pH were conducted by using a PHS-3C pH-meter (Dapu Instrumentation Co., Ltd. Shanghai, China). All the chemicals (Na5P3O10, K2S2O8, NaNO3, and Fe(NO3)3·9H2O) used in this study were of analytical reagent grade and the glassware used in the experiments were carefully cleaned with double deionized water. The NOM HA and FA were obtained from the soil of Jiufeng Mountain in Beijing city.
2.2. Goethite synthesis
Goethite was synthesized by following the procedures of Schwertmann and Cornell.17) In short, 45 mL of 5 M KOH was mixed with 25 mL of 1 M Fe(NO3)3 in a vessel, and the solution was quickly diluted with water to 1 L. The above suspension was then held in a closed polyethylene bottle at 70°C for 60 h. The resulting suspension was filtered and the goethite slurry washed with deionized water until the solution became pH neutral.
2.3. Characterization
An X-ray diffractometer (Bruker D8 Advance, Germany) equipped with a scintillation detector and a Cu Kα radiation source was employed to analyze the phase compositions of the samples in the 2θ range 10° to 70°. Infrared spectral analysis was performed by using a Nicolet 6700s spectrometer with KBr pellet technique with the width ranging from 400 to 4,000 cm−1. The morphology and structure of the samples were studied by scanning electron microscopy (JEOL JSM-6700F). The surface area was determined with an SSA-4200 BET surface area and pore size analyzer to be 28.62 m2·g−1. All the dried samples including the goethite before and after the adsorption of TPP, HA, FA, TPP+HA, and TPP+FA were milled with mortar and pestle to the required particle size.
2.4. Analytical methods
Prior to the determination of the TP, the solution was separated and filtered through a 0.22 μm micropore filter membrane to remove the solid particles. The concentration of polyphosphate in the solution was expressed in total phosphorus (CTP). The concentrations of TP were determined by the molybdenum blue method after digestion with K2S2O8.18) Total organic carbon (TOC) refers to the concentration of NOM (HA, FA) in the solution, which was determined with a TOC analyzer (Liqui TOC II, Elementar).
2.5. Adsorption experiment
TPP solutions with [TP] = 0-30 mg·L−1 were prepared by dissolving Na5P3O10 in deionized water. The amount of TPP was converted to [TP] in the following work. The removal of TPP was carried out by adding 0.05 g of goethite into a 50 ml solution, which was continued for 24 h. All the experiments were conducted at 293 K, the initial pH of 8.5, and the ionic strength of 0.01 M in a water bath that was agitated at 200 rpm. The kinetic experiment was performed in solutions with initial [TP] = 3, 5, and 10 mg·L−1 within 48 h. The isothermal adsorption experiment was carried out for initial [TP] = 1.5, 5, 10, and 15 mg·L−1 at 278, 288, and 293 K, respectively. The addition of HA and FA was carried out at initial [TP] = 5 mg·L−1. The order of addition is expressed as follows: Goe + TPP, HA refers to the case where Goe and TPP are first added, followed by HA; HA + TPP, Goe refers to the case where HA and TPP are first added, followed by Goe; Goe + HA, TPP refers to the case where Goe and HA are first added, followed by HA; and Goe + TPP + HA refers to the case of simultaneous addition of all the components.
3. Results and Discussion
3.1. Effects of ionic strength on TPP adsorption
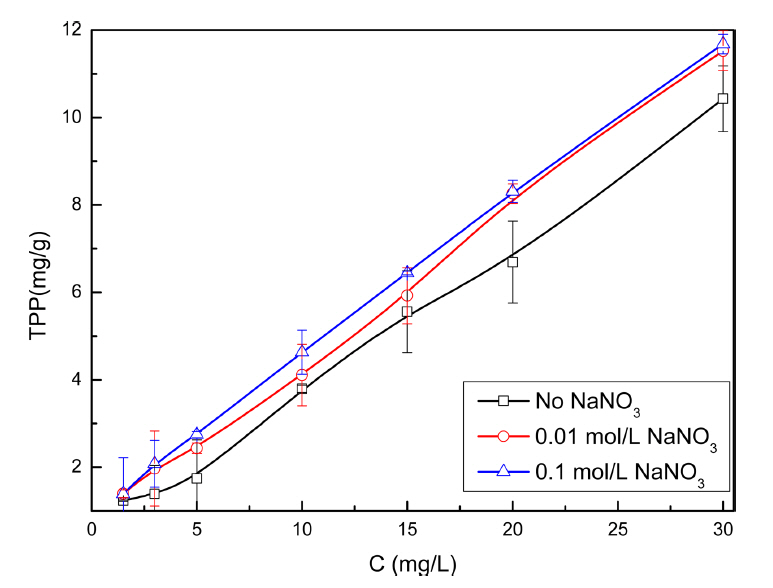
The effect of ionic strength on TPP removal was studied in NaNO3 solution. The experimental data are shown in Fig. 1. It clearly indicates that the adsorption of TPP on goethite obviously increases with increasing initial TPP concentration, which may be attributed to the sufficient number of active sites in goethite. The increase in the ionic strength can induce a less negative zeta potential at the adsorption plane of goethite, which can remarkably increase the TPP adsorption.19)
3.2. Effects of pH on TPP adsorption
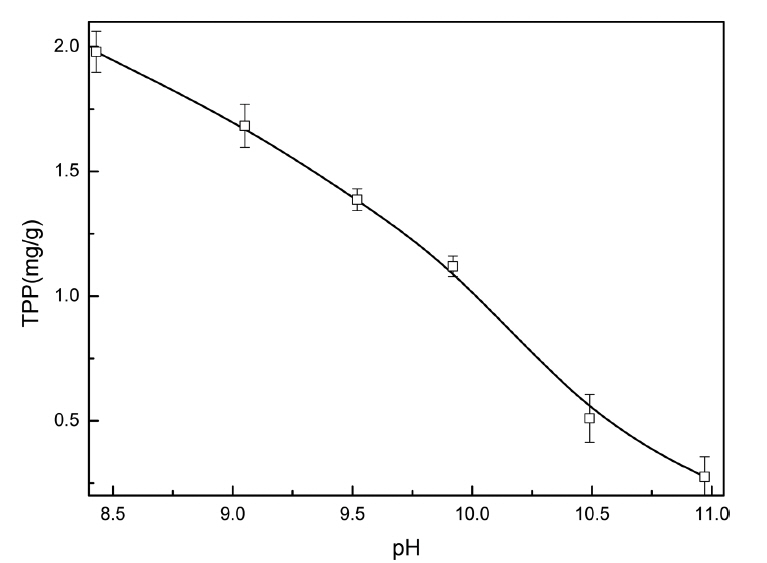
The pH is one of the important factors affecting the adsorption capacity. Since TPP is easily hydrolyzed in acidic conditions,20) this research studied the adsorption of TPP in alkaline solutions. The results revealed that the TPP adsorption capacity decreases significantly when the initial pH increases from 8.5 to 11.0 (Fig. 2). Since the point of zero charge of goethite is about 8.5,21) its surface accumulates more negative charge with the increase in the pH value and produces electrostatic repulsion between the negatively charged sites and the negatively charged TPP ions, thus significantly decreasing the adsorption capacity.22) In addition, the OH− in the solution compete with the TPP ions for the goethite adsorption sites, which may also contribute to the decreased adsorption of TPP.
3.3. Effects of temperature and adsorption isotherms
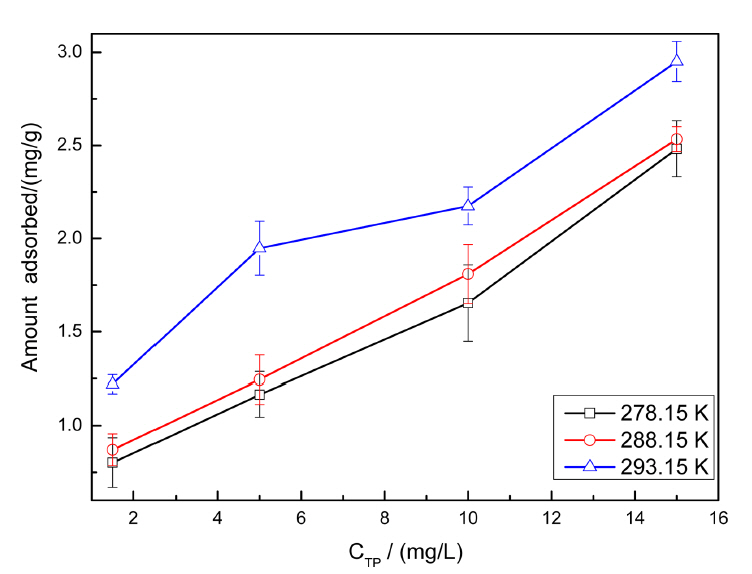
The equilibrium adsorption capacities obtained at different temperatures (278, 288, and 293 K) are shown in Fig. 3 and Table 1. The adsorption experiments were carried out at low temperatures to prevent TPP hydrolysis. Langmuir and Freundlich models were used to simulate the adsorption equilibrium data. The linear form of the isotherm model is written as23)
where Ce is the equilibrium adsorption concentration (mg·L−1), qe is the equilibrium adsorption capacity (mg·g−1), qm is the theoretical maximum monolayer adsorption capacity of the adsorbent (mg·g−1), b refers to the adsorption constant of Langmuir isotherm (L·mg−1), and KF and n are Freundlich constants related to the adsorption capacity and adsorption intensity, respectively. Table 1 summarizes all the adsorption isotherm parameters obtained at different temperatures; the Freundlich isotherm model revealed a higher correlation coefficient, indicating that the adsorption of TPP on goethite was heterogeneous and could be better explained by this model. The value of the constant KF increased with temperature, which indicated that the goethite adsorption was endothermic. The constant n varied between 2 and 10 for all the three temperatures, which indicated that adsorption was favored.24) In addition, according to Arrhenius equation (equation 3), the apparent activation energy of the process was 11.27 kJ·mol−1; the result also showed that the adsorption process included chemical adsorption.
In the Arrhenius equation, K is the reaction rate constant (g·mg−1·min−1), A is the frequency factor, Ea is the apparent activation energy (kJ·mol−1), R is the molar gas constant (J·K−1·mol−1), and T is the reaction temperature (K).
3.4. TPP adsorption kinetics
The experimental results of the TPP adsorption kinetics are shown in Fig. 4 and Table 2. The three different adsorption curves suggested that the process occurred faster within the first hour, before becoming slower as the adsorption time increased. Therefore, the adsorption equilibrium time was determined to be less than 24 h. According to the equilibrium adsorption capacity, the pseudo-first-order and pseudo-second-order equations were used to explain the adsorption mechanism of TPP removal:
where qt (mg·g−1) is the adsorption capacity at the contact time t (h), qe is the equilibrium adsorption capacity (mg·g−1), k1 (min−1) is the pseudo-first-order rate constant, and k2 (g·mg−1·min−1) is the pseudo-second-order equilibrium rate constant.
The parameters of the pseudo-first-order and pseudo-second-order models were calculated based on the experimental data and the results are presented in Table 2. It was observed that the correlation coefficients were very close to 1.0 and that the calculated adsorption capacity qe(cal) of the pseudo-second-order model agreed well with the experimental equilibrium adsorption capacity qe(exp). The pseudo-firstorder equation only described the initial stage (the first 10 min) of the adsorption, whereas the pseudo-second-order equation could well predict the entire adsorption process.
3.5. Effects of HA and FA
The TPP adsorption capacities of goethite with or without HA are shown in Fig. 5. The higher the concentration of HA added to the system (increasing from 0 to 100 mg·L−1), the lower was the amount of TPP adsorbed (decreased from 2.41 to 1.14 mg·g−1), which indicated that both TPP and HA competed for adsorption on the goethite surface. Differences in the addition orders of TPP, HA, and goethite resulted in minimal changes to the goethite adsorption capacity. The adsorption capacity of Goe + TPP, HA > that of Goe + HA, TPP, which indicated that HA could not outdo the TPP adsorbed on the surface of goethite. In addition, HA was strongly bonded to goethite, and was not easily replaced by TPP either.
For a comparison, the effect of FA on TPP adsorption is also presented. The data are displayed in Fig. 6, where the adsorption capacity increased obviously (from 2.41 to 3.49 mg·g−1) after the addition of different amounts of FA (from 0 to 100 mg·L−1) into the mixed solution of goethite and TPP; on the other hand, the adsorption capacity showed little change when TPP was added to the mixed solution of goethite and FA. The reason might be that TPP not only was bound directly to the sorption sites in goethite but also formed FA-TPP-iron complexes, which then coated on the surface of goethite to increase the sorption capacity of TPP on goethite.25,26)
3.6. Mechanism
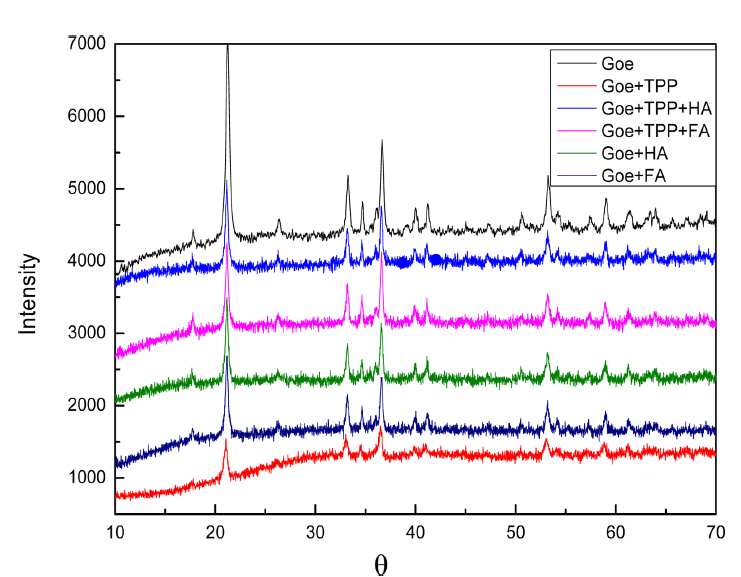
The X-ray diffractograms of goethite before and after the adsorption of TPP are presented in Fig. 7.
The peaks coincided well with those of pure goethite, which revealed that the adsorption did not change the structure of the inner goethite. However, the intensities of those XRD peaks decreased, which was possibly due to a portion of the XRD signal being related to the adsorbed organic matter, which suppressed the signal corresponding to goethite.27)
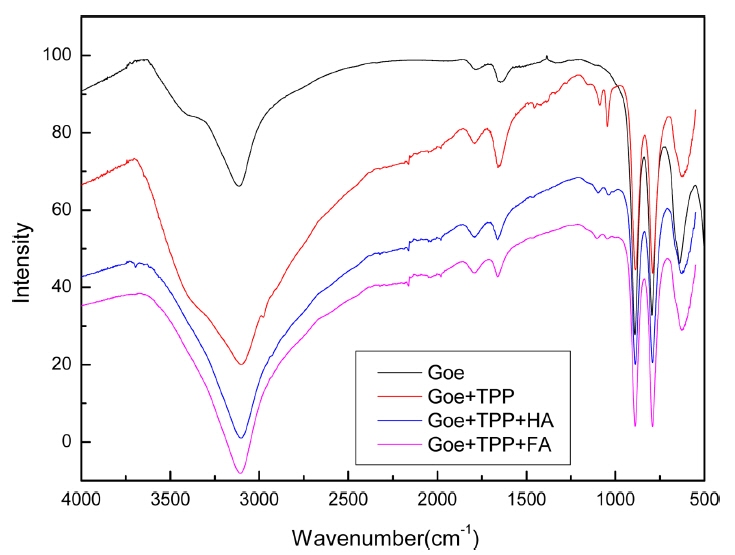
The IR spectra of unloaded and loaded goethite are shown in Fig. 8. Compared to that of unloaded goethite, the IR spectrum of loaded goethite revealed two bands (1,044 and 1,087 cm−1), which were assigned to the overlapping asymmetric (1,087 cm−1) and symmetric (1,044 cm−1) stretchings of the terminal PO43−.28) The appearance of new peaks and the change in intensity in the FT-IR spectra at different adsorption conditions suggested that the adsorption process may form a new complex.
Figure 9 shows the SEM images of goethite before and after the adsorption of TPP and HA/FA. Goethite before being treated with phosphate (Fig. 9(a)) exhibited long, acicular (needle-like) crystals and a more regular surface. Each goethite particle after adsorption showed aggregation. The samples prepared with TPP and FA illustrated not only the aggregation but also the formation of a light grey film on the surface of goethite, which might be attributed to the reaction of TPP and goethite-FA complexes.
4. Conclusions
In the current work, the removal of TPP by using synthetic goethite was investigated systematically. It was found that the adsorption capacity decreased obviously with increasing pH, but changed little with increasing electrolyte concentration. As for the results of the kinetic studies, the absorption capacity quickly increased within 1 h and equilibrium was reached within 24 h. The adsorption results of this study fitted well with the pseudo-second-order rate equation and Freundlich model. Different orders of addition of TPP, HA, and goethite resulted in different adsorption capacities of goethite: Goe + TPP, HA ≈ Goe + TPP + HA ≈ HA + TPP, Goe > Goe + HA, TPP. Subsequent addition of FA could increase the TPP absorption capacity of goethite, which might be attributed to the adsorption of TPP on FA. Characterization of the materials showed no change in the internal goethite, which revealed that the adsorption mechanism may be mainly based on surface complexation and electrostatic adsorption.