1. Introduction
The two key requirements for advanced lithium-ion batteries (LIBs) are higher power and energy densities, which are crucial factors for achieving quick-charge and long lifespan for a single charge, respectively.1-4) From this viewpoint, researches have focused on developing alternative electrode materials with high rate performance and reversible capacities. Ge, a group IV element, has therefore been considered as one of the most promising anode materials for LIBs.4-12) Although its theoretical capacity (1600 mA h g−1) does not match up to that of Si (4200 mA h g−1), Ge has many advantages over Si. Compared with those of Si, the Li ion diffusivity of Ge is 400 times higher and the electrical conductivity is 104 times higher.12-20) However, similar to Si, Ge suffers from drastic volume changes during lithiation and delithiation. This results pulverization of the electrode, which leads to huge capacity fading.18-25) In light of these concerns, various strategies for buffering such huge volume changes have been proposed based on the introduction of rationally designed nanostructures.
For example, Park et al. prepared Ge nanotubes by exploiting the Kirkendall effect.9) Wang et al. synthesized Ge nanoparticles by magnesiothermic reduction.18) Gu et al. demonstrated the template-free preparation of a Ge nanowire film electrode.26) Incorporation of Ge with carbon is another attractive strategy as carbon confers improved stability to the resulting Ge-C composite by hindering aggregation of Ge during repeated cycling.27-33) For instance, Gao et al. synthesized a Ge/graphene carbon nanotube hybrid.30) Xu et al. reported Ge nanoparticles encapsulated in N-doped reduced graphene oxide.31) Both of these species showed stable cycling performance over 200 cycles.
Spray pyrolysis, which is a simple and scalable process that is used for the preparation of various nanocomposites, can also be efficiently applied to the synthesis of Ge-C composites. In this study, we prepared Ge nanoparticle-dispersed reduced graphene oxide (rGO) balls by simple reduction of a GeO2-rGO balls obtained by spray pyrolysis. rGO serves as a robust buffer for the volume changes during lithiation/delithiation and hinders aggregation of Ge. In addition, hybridization of rGO with Ge leads to greatly improved electrical conductivity. The enhanced Li-ion storage performance of Ge-rGO is demonstrated relative that of Ge metal powder.
2. Experimental Procedure
2.1. Sample preparation
The Ge nanoparticle-dispersed reduced graphene oxide (rGO) (Ge-rGO) balls were prepared by spray pyrolysis and H2 gas reduction. The spray pyrolysis process is thoroughly described in our previous report.24) The spray solution was prepared by dissolving 2.09 g of GeO2 (99.999%, Junsei) in distilled water with ammonia (NH4OH, Samchun) under mild heating. Graphene oxide (GO) was obtained by a modified Hummer’s method. The as-obtained GO was redispersed (1 mg mL−1) in the above solution and sonicated for 1 h. The total volume of the colloidal solution was 500 mL. The temperature of the furnace was fixed at 500°C. The solution was atomized and carried by N2 gas at a flow rate of 10 L min−1. An appropriate amount of as-synthesized powder was transferred to the tubular furnace and heat-treated at 450°C for 1 h with 10% H2/Ar gas to obtain the Ge-reduced graphene oxide (Ge-rGO) composite powder. For comparison, Ge metal powder was prepared by reducing commercial GeO2 powder at 450°C for 3 h.
2.2. Characterization
Scanning electron microscope (SEM; TESCAN, VEGA3) and field-emission transmission electron microscope (TEM; JEOL, JEM-2100F) were employed to observe the morphologies of the samples. X-ray diffraction (XRD; X’Pert PRO MPD) measurements were conducted with Cu-Kα radiation. Thermogravimetric analysis (TGA; SDT Q600) was conducted in air with a ramping rate of 10°C min−1 to estimate the amount of carbon in the sample.
2.3. Electrochemical measurements
The lithium ion storage properties of the samples were characterized by assembling 2032-type coin cells. The detailed electrode preparation method and cell configuration are thoroughly described in our previous report.34) The cell was cycled over the potential range of 0.001-3 V at various current densities. Cyclic voltammetry was conducted at a scan rate of 0.1 mV s−1. The electrochemical impedance spectroscopy (EIS) was conducted over the frequency range of 0.01 Hz-100 kHz by the potentiostat/galvanostat instrument (ZIVE SP1, WonA Tech).
3. Results and Discussion
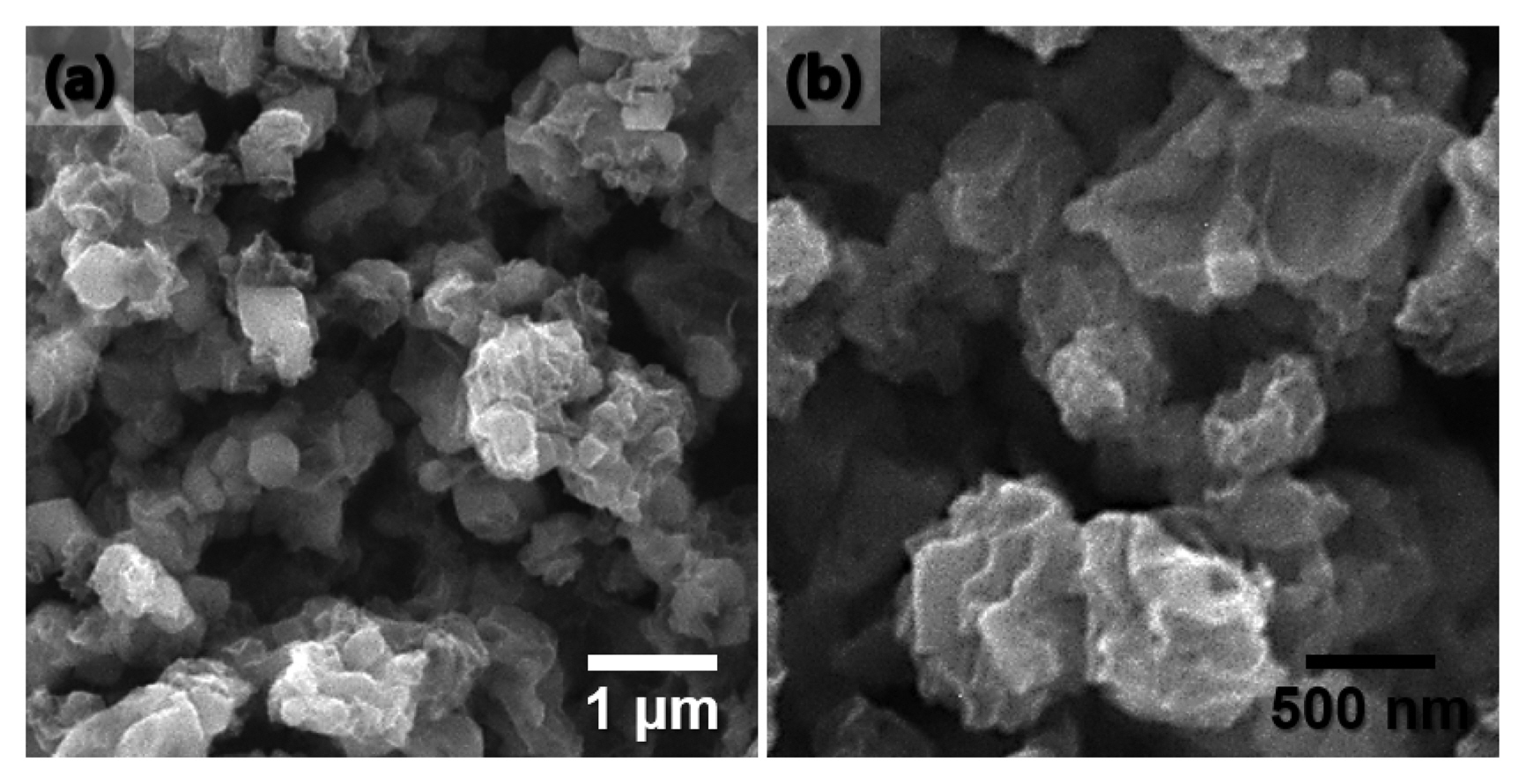
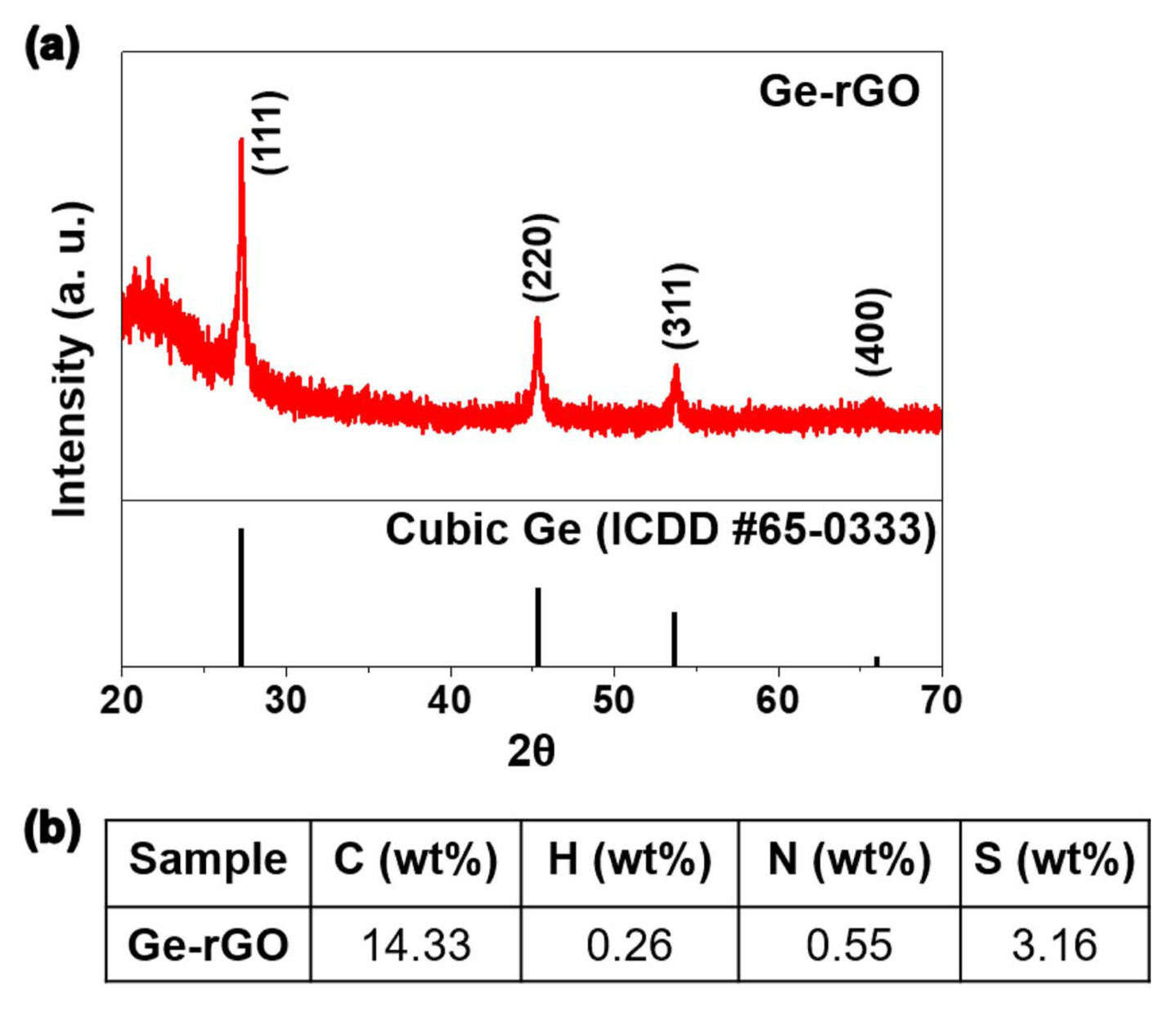
The SEM images of the GeO2-rGO balls prepared by spray pyrolysis are shown in Fig. 1. The balls are crumpled and irregularly shaped. The morphologies of the Ge-rGO balls obtained by heat treatment under reducing atmosphere are shown in Fig. 2. The SEM and TEM images in Figs. 2(a) and (b) confirm that the original morphology of the powder was maintained and no crystal growth of Ge was occurred. A clear distinction between the Ge nanocrystals and rGO sheets was observed in the high-resolution TEM image in Fig. 2(c), and the lattice image in the inset of Fig. 2(c) reveals fringes separated by 0.33 nm, which correspond to the (111) plane of cubic Ge. The selected-area electron diffraction (SAED) pattern and XRD pattern, respectively presented in Figs. 2(d) and 3(a), also confirmed the successful formation of cubic Ge. The elemental mapping images in Fig. 2(e) demonstrate the homogeneous distribution of C and Ge throughout the composite, indicating successful incorporation of the germanium nanocrystals and rGO sheets.
CHNS analysis was conducted to estimate the exact amount of the rGO content in Ge-rGO powder (Fig. 3(b)). According to the result, the carbon content of Ge-rGO was 14.3 wt%. Slight amount of N and S components were also detected, which were likely to be derived from the ammonia solution and sulfuric acid that were used to dissolve GeO2 and prepare GO solution, respectively.
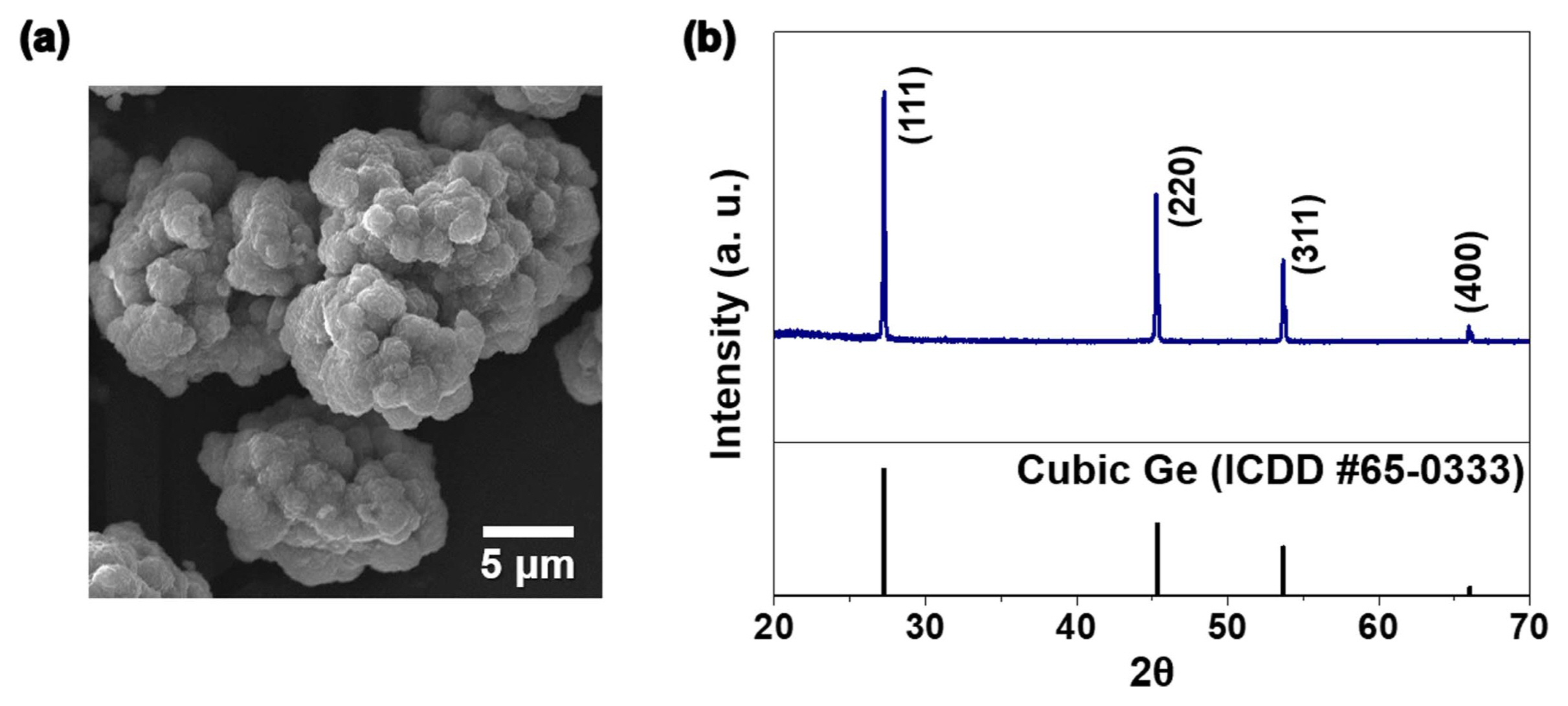
The Li-ion storage performance of the Ge-rGO composite powder was compared with that of Ge metal powder obtained by reducing GeO2 powder. The SEM image and XRD pattern of the Ge metal powder presented in Figs. 4(a) and b indicate the formation of highly crystalline Ge metal after reduction. The CV curve of Ge-rGO in the range of 0.001-3.0 V at a scan rate of 0.1 mV s−1 is shown in Fig. 5(a). In the first cathodic scan, reduction peaks appeared at 0.55, 0.36, 0.19, and 0.06 V. The peak at 0.55 V is attributed to the formation of a solid electrolyte interphase (SEI) layer.14,30,31) This peak was not observed in the subsequent cycles, indicating the irreversibility of the reaction. The other peaks at lower potentials were attributed to the formation of LixGe alloys such as Li7Ge2, Li9Ge4, and Li22Ge5 intermediates.28,30,35-37) The large peak at 0.06 V also corresponded to the reversible Li-ion insertion into graphene.30,38) From the second cycle onward, these reactions were observed at 0.41 and 0.09 V. The oxidation peaks at 0.54 and 0.73 V in the anodic scan are ascribed to the delithiation reaction of the Li-Ge alloy. The small peak at 1.12 V was ascribed by the oxidation of Ge to GeOx.24,39) This indicates that Ge nanoparticles on the rGO sheets were partially oxidized, since nanosized Ge crystal is more susceptible to the oxidation. From the second cycle onward, the curves overlapped, indicating good reversibility and stability of the reaction. Overall, the entire lithiation/delithiation reaction can be summarized as follows:15)
Figure 5(b) shows the CV curves of Ge metal. Compared with that of Ge-rGO, the first cathodic scan of Ge metal shows one large peak at 0.07 V, which indicate the lithiation reaction.18) Three peaks were observed at 0.46, 0.35, and 0.13 V in the subsequent cycles, indicating the multi-step lithiation reactions, as discussed above. The anodic peak at 0.63 V corresponds to the delithiation reaction.15,36) From the second cycle onward, however, the intensities of the cathodic and anodic peaks diminished, indicating the instability of the electrode, mainly caused by pulverization during cycling. The initial discharge-charge profiles of the Ge-rGO and Ge metal powders are shown in Fig. 5(c). The discharge profile of Ge-rGO exhibited a distinctive plateau at roughly 0.5 V, which matches to the reduction peak observed in the CV curve in Fig. 5(a). Similarly, the discharge profile of Ge metal powder exhibited a very distinctive plateau at 0.25 V that corresponds to the sharp reduction peak in Fig. 5(b). The charge profiles of the Ge-rGO and Ge metal powders showed a plateau around 0.3 and 0.5 V, which also coincided with the oxidation peaks observed in the CV curves in Figs. 5(a) and (b). The initial discharge capacities of the Ge-rGO and Ge metal powders were 1443 and 1514 mA h g−1, respectively, and the corresponding charge capacities were 996 and 1341 mA h g−1. The low initial Coulombic efficiency of Ge-rGO resulted from the large irreversible capacity caused by decomposition of the electrolyte and formation of the SEI layer, which is generally affected by the surface area of the electrode. The cycling performance of the Ge-rGO and Ge metal powders at 1.0 A g−1 are presented in Fig. 5(d). The reversible discharge capacity of Ge-rGO was fairly stable over 200 cycles (748 mA h g−1), whereas that of Ge metal faded seriously after a few cycles, exhibiting the discharge capacity of merely 78 mA h g−1 at the 200th cycle. The rate performance of the Ge-rGO and Ge metal powders is summarized in Fig. 5(e). The discharge capacities of Ge-rGO at current densities of 0.5, 1.0, 2.0, 3.0, 4.0, and 5.0 A g−1 were 980, 883, 775, 702, 634, and 375 mA h g−1, respectively. On the other hand, the discharge capacities of Ge metal at the corresponding current densities were 937, 747, 542, 332, 232, and 160 mA h g−1. The superior electrochemical performance of Ge-rGO relative to that of Ge metal could be ascribed to the synergistic effect between the Ge nanocrystals and rGO. Incorporation of the Ge nanocrystals with the rGO sheets effectively reduced the mechanical stress induced by the volume change of Ge during lithiation and delithiation, thus preventing pulverization of the electrode.16,23,27) In addition, the open channel between the rGO sheets facilitated penetration of the electrolyte, which in turn provided ample sites for reaction of the Li ions with the Ge nanocrystals. Furthermore, the presence of rGO improved the electrical conductivity of the powder, contributing to the enhanced rate performance.14,30,31) EIS analysis was employed to confirm the superior electrochemical performance of Ge-rGO relative to that of Ge metal powder. The semicircle in the high-to-medium frequency region represents the charge-transfer resistance (Rct) of the electrode. The Nyquist plots of the Ge-rGO and Ge metal powders from the half-cells, which were obtained after 50 cycles with fully charged state, are presented in Fig. 5(f). The plots were obtained by deconvolution with a Randle-type equivalent circuit model shown in Fig. 5(g). The electrochemical reaction types such as charge transfer reaction, lithium ion migration through SEI layers, and Li+ ion diffusion kinetics in the active materials are described in the equivalent circuit model. The diameter of the semicircular region for Ge-rGO in Fig. 5(f) was smaller than that for Ge metal. The precise values of charge transfer resistance of Ge-rGO and Ge metal powders were acquired from the simulated equivalent circuit. Accordingly, the Rct values for the Ge-rGO and Ge metal powders were 10 and 48, respectively. The low Rct of Ge-rGO is attributed to its structural stability and high electrical conductivity, which is in accord with the superior cycling stability and rate performance.30,40)
4. Conclusions
In this study, the enhanced electrochemical properties of a Ge-rGO powder as anode material for LIBs were confirmed. Successful incorporation of Ge and rGO nanosheets was achieved by simple spray pyrolysis and subsequent H2 reduction. The synergistic effect between the robust, highly conductive rGO nanosheets and Ge improved the cycling stability and rate performance of the resulting composite. Thus, Ge-rGO powder could exhibit a stable reversible discharge capacity over 200 cycles and good rate performance at a high current density of 5 A g−1.