1. Introduction
The enforcement of regulations on environmental pollution caused by industrialization has highlighted the need for qualitative tools to detect the release of toxic gases and their concentrations. While gas chromatography is usually employed in odor and gas analysis, this method involves expensive equipment that is not easy to transport or operate. The development of low-cost and objective gas sensors as an effective method of detecting toxic or combustible gases and measuring their concentrations based on physical and chemical properties has been attracting interest.
Gas sensors can be largely categorized into semiconductor gas sensors, solid electrolyte gas sensors, and catalytic combustible gas sensors.1-3) Semiconductor gas sensors are known for their outstanding sensitivity and response rate; they last longer than other types of gas sensors. These sensors make use of the change in electrical conductivity that occurs when a semiconductor surface comes into contact with gas. The change is electrical conductivity is induced by the varying of the width of the depletion layer due to the movement of electrons when a gas is adsorbed onto the semiconductor surface.4,5) In 1962, Seiyama et al. proposed a metal oxide (MOx) semiconductor gas sensor using ZnO2 films, and this has led to extensive research on semiconductor gas sensors to this day.6)
Gas sensors are classified into n-type or p-type depending on the change in direction of conductivity when exposed to a reducing gas. N-type gas sensors have electrons as their majority charge carriers, and show an increase in conductivity upon reacting with a reducing gas. On the other hand, p-type gas sensors have positive holes as their majority charge carriers, and exhibit an increase in conductivity upon reacting with an oxidizing gas. Tellurium (Te) shows p-type conduction because its lattice defects act as acceptors. 7) Te has a band gap of 0.34 eV, a carrier concentration of (1 - 5) × 1018 cm−3 at room temperature, and a Hall mobility of 20−5 cm2/Vs.8) According to some studies, Te-based gas sensors have fast response and satisfactory sensitivity in the range of ppm and sub-ppm concentrations.9,10) Their flexible structure and high compatibility with alloys allow the material to assume different properties under various conditions, and sensors can be designed to suit a wide range of gases.
Te, which is a key element of thermoelectric materials, has seen an increase in demand with industrialization and is widely used in many fields (thermoelectric semiconductor, PV cell, catalyst etc.).11) The rapid increase in demand since the 2000s has resulted in unstable prices of thermoelectric materials, and supply has grown limited due to restrictions imposed by resource-rich countries.12) Because Te-based compounds are only available as imports in some countries and have an extremely low rate of reusability, it is necessary to cut down on costs from the material stage by developing recycling technology and more economic processes.13) In spite of this, few studies exist on the recovery of thermoelectric materials from thermoelectric scrap and on their integration in new applications. This study recovered thermoelectric scrap using a wet reduction process and, to analyze the gas sensing properties, created a Te paste from separated Te powder.
2. Experimental Procedure
2.1. Recovery of Tellurium
The particles to be used in examining gas sensing properties were prepared as follows. After heating the waste thermoelectric modules at 250°C, soldered n-type (Bi2Te3) and p-type (Bi0.5Te1.5Te3) chips were separated from the modules. To free the chip surface of residual solder, the separated chips were placed in HCl (37%, Sigma-Aldrich Co., Ltd., USA) for six hours.
For classification of materials into n-type and p-type, 0.14 mmol of chips were each immersed in 30 mL of solution, comprised of HNO3 (70%, Sigma-Aldrich Co., Ltd., USA) and H2O in the ratio of 2 to 1, and dissolved for 24 h. A syringe filter was used to extract solid p-type chips and to remove impurities existing in the recovered solution.
NaOH (98%, DAEJUNG) was added to the filtered solution to adjust the pH to 13. At pH 13, 3 ml of N2H4·H2O (100%, ACROS ORGANICS) was added, and reduction reactions were allowed to occur for 12 h at 70°C. Once reactions were complete, a centrifuge and syringe filter were used to obtain an aqueous solution containing Te ions, and particles were retrieved in the form of dried powder. HNO3 was again used to adjust the pH of the Te-containing solution to 7, and 2.184 g of cetyltrimethylammonium bromide (CTAB) was added. Next, N2H4·H2O was added for re-reduction at 70°C. The resulting powder was dried after washing with ethanol and DI water, and analyzed using X-ray diffraction (XRD), field emission scanning electron microscopy (FE-SEM) and energy dispersive X-ray spectroscopy (EDS). Te powder was used to examine the gas sensing properties.
2.2. Sample Analysis
The crystal structure of the resulting powder was subject to XRD (ULTIMA IV, Rigaku) analysis, with Cu Kα (λ = 0.154 nm) radiation at 40 kV, 40 mA. The particle shapes were analyzed using FE-SEM (S-4300, Hitachi) and, at the same time, elemental analysis was performed through EDS (EDAX Pegasus 4040, EDAX). Concentrations were varied by controlling the flow of the target gas and air with a mass flow controller (Alicat, MC-500SCCM-D); sample resistance under varying concentrations was measured in real-time with a multimeter (Keithley-2000).
2.3. Preparation and Measurement of Gas Sensor Properties
To assess the gas sensing properties of Te powder obtained through re-reduction, Te paste was prepared by mixing the Te powder with DMF solvent and ethyl cellulose binder. Te paste was handprinted on Si wafers containing SiO2 layers with interdigitated Au electrodes deposited at an interval of 200 μm; samples were then dried for 24 h at 80°C. Measurements were taken after stabilizing the resistance of samples at room temperature. The total flow of NOx gas and air was fixed at 500 sccm, and measurements were obtained using a mass flow controller to vary the concentration of NOx gas from 10 ppm to 50 ppm.
3. Results and Discussion
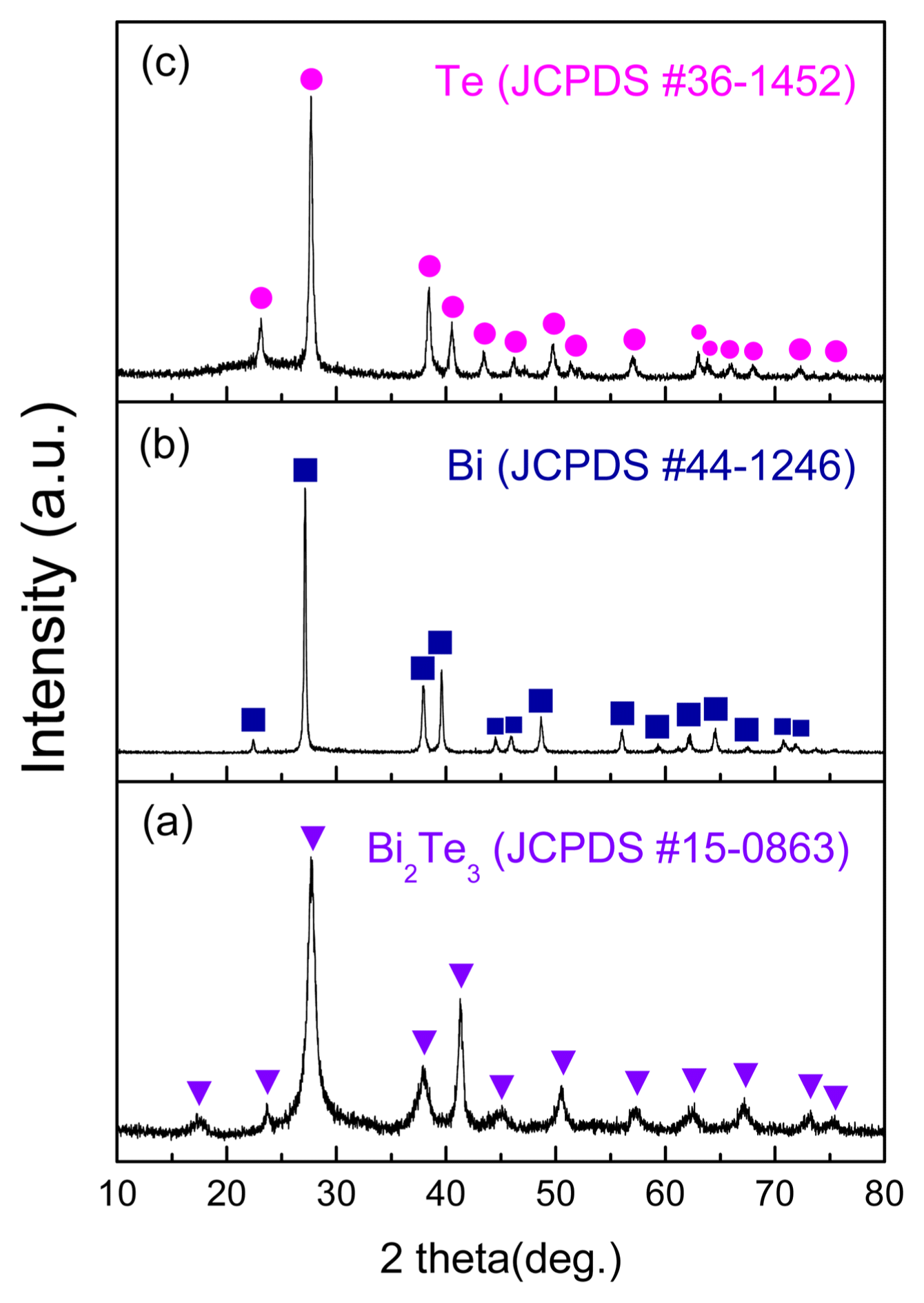
Figure 1 shows the results of XRD analysis for the powder prepared with a precursor solution in which n-type chips were dissolved. Fig. 1(a) shows the XRD patterns of samples prepared by adding hydrazine in the absence of NaOH. Diffractions of (015), (1010), and (110) were observed at 27.6°, 37.8°, and 41.1° respectively. Both Bi and Te ions reacted with samples not containing NaOH, and formed a Bi2Te3 hexagonal crystal structure (JCPDS #15-0863) having an R3̄m (166) space group. Fig. 1(b) shows the results for samples reduced after adding NaOH and adjusting the pH to 13. Diffractions of (100), (101), (102), and (110) were observed at 23.0°, 27.5°, 38.2, and 40.4°, respectively. When hydrazine is added at pH 13, the reduction process is centered around Bi because, at this pH, Te exists as TeO3 2− and Bi as Bi2O3. The reduction of Bi2O3 takes place with hydrazine acting as a strong reducing agent. The Bi hexagonal crystal structure (JCPDS #44-1246) was found to contain the R3̄m (166) space group. Fig. 1(c) shows the results for samples that have undergone re-reduction after adding NaOH to obtain a pH of 7. Diffractions of (100), (101), (102), and (110) were observed at 23.0°, 27.6°, 38.3°, and 40.4°, and the Te hexagonal crystal structure (JCPDS #36-1452) was found to contain the P3121 (152) space group. The equations below express the behavior and reduction reaction of Bi at pH 13.
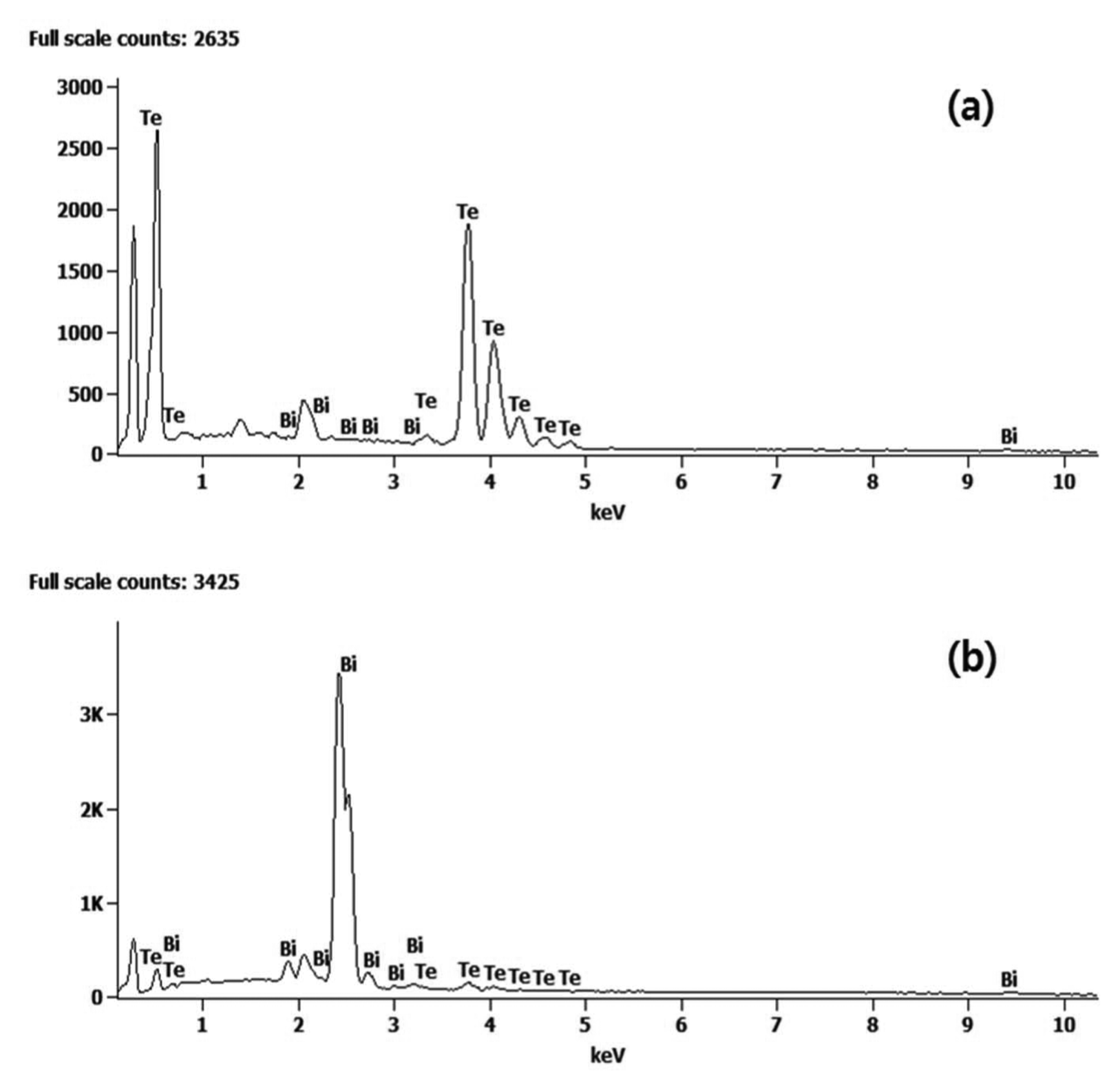
The powders prepared at different levels pH were analyzed, as shown in Fig. 2, using EDS. Both Te and Bi were detected. To check for the separation of Te from Bi, the analysis excluded carbon and oxide, focusing only on Bi and Te. The peak exhibited at 0.175 keV is the C peak. In Fig. 2(a), Te peaks can be clearly seen at 0.46 keV, 3.75 keV, 4.03 keV, and 4.31 keV, and these results are consistent with the Te EDX results. Some Bi peaks were also analyzed. Most peaks in Fig. 2(b) are peaks of Bi; peaks matching Bi were observed at 2.04 keV, 2.43 keV, and 2.71 keV. Similar to the previous samples, some Te peaks were included in the analysis. Table 1 presents the results of quantitative analysis in wt.% and at.% for Te and Bi.
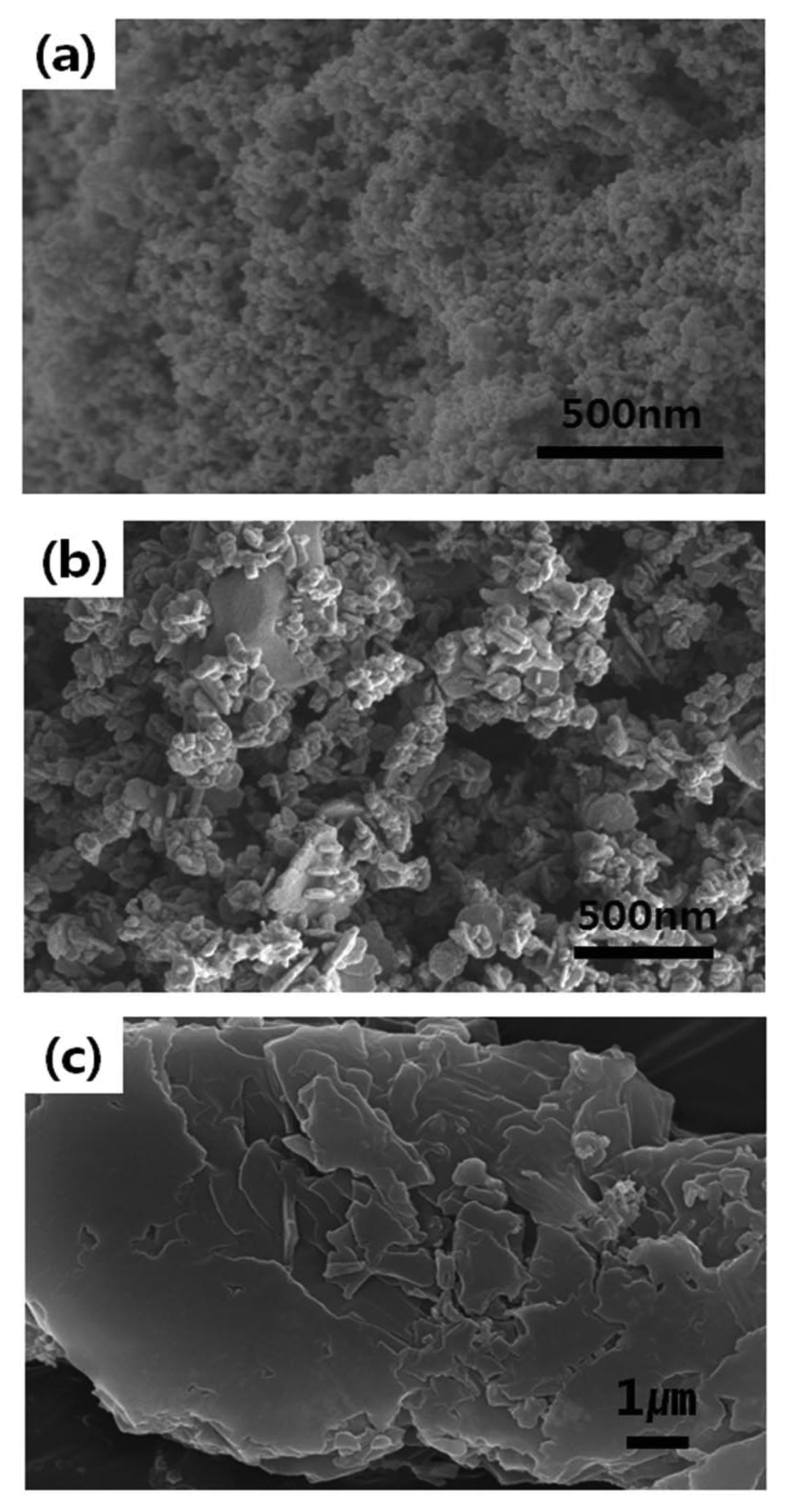
Figure 3 gives the FE-SEM results for particles prepared under varying pH conditions. The samples in Fig. 3(a), which have been reduced after adding NaOH but without changing pH, have spherical particles. As shown in Fig. 3(b), the samples reduced at pH 13 have irregular particles. The particles in Fig. 3(c) were obtained after adding CTAB and performing reduction at pH 7. These samples have distinct shapes and are more coarsely coagulated compared to the other samples.
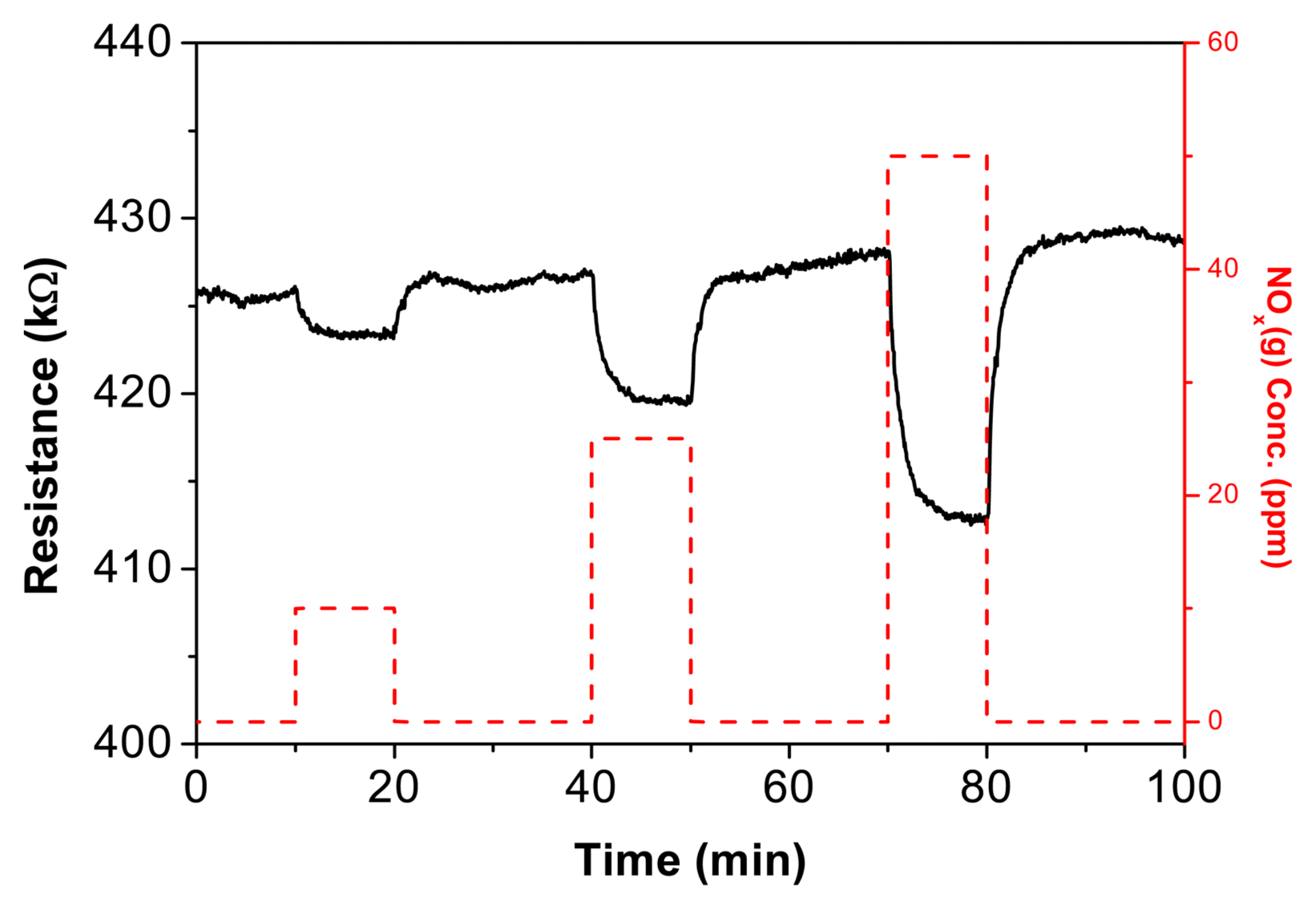
Figure 4 shows the NOx gas sensing properties of Te paste recovered from printing CTAB on interdigitated Au electrodes. The concentrations of NOx in the gas chamber were set at 10 ppm, 25 ppm, and 50 ppm. Table 2 presents the response rate (T90: time needed to reach 90% of resistance after gas exposure), recovery rate (D10: time needed to recover 10% of current signal value after removing gas), and sensitivity (Ra/Rg, Ra: resistance of sample in atmosphere, Rg: resistance upon exposure to target gas) at different concentrations. Sensitivity increased linearly as NOx(g) concentration rose from 10 ppm to 50 ppm, and reaction and recovery rates fell below three minutes. The decreasing resistance of the Te paste upon interaction with the oxidizing gas NOx(g) indicates an increase in the number of positive holes as charge carriers in Te; this material exhibits the properties of p-type semiconductors.
4. Conclusions
This study dissolved waste thermoelectric modules in an HNO3 aqueous solution and adjusted the pH to separate Bi and Te particles using a wet reduction process. The powders were prepared under different conditions, and XRD analysis revealed a single spectrum of Bi at pH 13 and of Te at pH 7. Under EDS analysis, Bi was found to constitute most of the reduced particles at pH 13, while Te with 99% purity was observed in the reduced particles at pH 7. These results confirm that elements can be easily separated for n-type (Bi2Te3) conductors through pH adjustments. The Te particles, after separation from Bi, were integrated into a new application. Gas sensing properties were determined under NOx gaseous atmosphere with the Te paste, which was prepared by mixing Te powder with DMF solvent and ethyl cellulose binder. The sensitivity, reaction rate, and recovery rate increased in proportion with the gas concentration. The paste exhibited a fast reaction rate and a recovery rate of less than three minutes at various concentrations.