1. Introduction
Vanadate glass is characterized with a large glass forming area and semi-conducting property due to the inherent characteristic of having various oxidation states simultaneously. Applications in a wide range of industries including semiconductor switching devices, electrochemical batteries, and sensors are possible utilizing such properties, garnering interest in this material as a new source of technology in the field of semiconducting glass.1,2) In the case of glass mixed with two or more transition metal oxides among such semiconducting oxide glasses, numerous studies were carried out to identify intermetallic ion mixed exchange pairs. Bogomolova et al. discovered the formation of a Cu2+-V4+ pair between the different transition metal ions V and Cu in glass with a composition including a mix of two or more transition metals such as BaO-V2O5-CuO and P2O5-CaO-V2O5-CuO; it was reported that a strong exchange interaction existed between the Cu2+ and V4+ ions.3) Also, with regard to the transition metal and electrical property, Mansour et al. found that the formation of a mixed pair between different transition metal ions influenced the electrical property of vanadate glass because electron hopping could occur between the intermetallic ions.4,5) It was predicted that the Fe3+ ion in the Fe2O3-V2O5 glass can combine to both interstitial and substitutional forms in the V2O5 glass matrix; the V and Fe ions exist in a mix according to the crystallization, in which new combinations occur to form bond pairs that affect the electrical conductivity and catalytic properties.6,7)
Most transition metal oxides form non-stoichiometric bonding with oxygen because the metal ions exist in various oxidation states. Oxygen deficiency occurs within a non-stoichiometric chemical compound due to oxygen defects; structural deficiency from such oxygen deficiency and transition metal oxide defects significantly impact the catalytic property. 8) Additionally, matrix defects like oxygen defects act as active sites for selective oxidation, ammonia oxidation, and dehydrogenation. As a result, the catalytic activity of nanoparticles created from transition metal oxides causes defects like oxygen defects and facilitates effective oxygen transport of materials through oxygen deficiency and enhancing of the catalytic ability; oxygen defects become active sites for organic bond (C-H) decomposition.9,10) In addition, the electrical conductivity of glass is not dependent only on the polaron radius and redox ratio V4+/(V4++V5+); the glass structure also plays an important role in influencing the electrical property.4)
In this study, heat treatment time for 70V2O5-10Fe2O3-13P2O5-7B2O3 glass including two transition metal oxides was varied and crystallization was carried out to perform crystalline phase analysis; thermogravimetric analysis (TGA) was used to investigate the catalytic ability for the oxidation reaction of fatty acids. The glass structure change was predicted to affect the catalytic property and electric conductivity and induce change; density and molar volume measurements, X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), and Fourier transform infrared (FT-IR) spectroscopy were used to investigate the causes and mechanisms behind the property changes through qualitative and quantitative analyses of the glass structure.
2. Experimental Procedure
2.1. Glass fabrication and crystallization
In this study, the starting materials used for the fabrication of 70V2O5-10Fe2O3-13P2O5-7B2O3 glass were Extra Pure grade V2O5, Fe2O3, NH4H2PO4, and B2O3 of Junsei Chemical Co., LTD. After mixing the substances for 10 min in an alumina mortar according to the composition ratio, the mixture was placed in an alumina crucible and melting was carried out for 2 h at 800°C in an electric furnace; then, the mixture was rapidly cooled in regular atmosphere on top of a stainless steel plate. The fabricated glass was grinded using an automatic grinding mixer and passed through 325 mesh. The filtered powder (d < 44 μm) was assessed through DSC, XRD, FTIR, XPS, and TGA analyses. The glass specimen used for the electrical conductivity, density, and molar volume measurements was cut into dimensions of 10 × 10 mm; SiC paper (# 220-1000) was used to fine polish the specimen so that it had parallel sides and a thickness of 2 mm.
DSC (SDT Q600, TA instrument, USA) was used to identify the glass transition temperature (Tg) and crystallization peak temperature (Tp), as shown in Fig. 1. Also, after annealing at a temperature of Tg or higher for 1 h on a carbon mold for the glass crystallization, heat treatment was carried out for 6 and 12 h at crystallization temperatures of Tp1 and Tp2, respectively.
2.2. Material property evaluation
In order to assess the structural change according to the crystallization, the density and molar volume were measured. The density of the glass specimen was measured using the Archimedes method (GH-200, AND Co, Korea) by placing samples with dimensions of 10 × 10 × 2 mm under water. The molar volume was then calculated using the measured density and the equation (VM ≡ &Mmacr;/ρ &Mmacr;: Average molecular weight of the glass). The average measurement value of 3 measurements was calculated and used as the measured density value; error bars and standard deviation of the experiments were indicated. Also, for the crystalline phase analysis, Ultima 4, manufactured by Rigaku, was used to determine the presence of (Cu-Kα) crystallinity in the 2θ range of 10-80° with step sizes of 0.02°; the type of crystalline phase formed was also determined.
In order to evaluate the glass structure change, a Spectrum GX (Perkin Elmer Co, USA) spectrometer was used to carry out FTIR analysis. KBr (Mixing ratio 1: 1200) powder was fabricated by preparing a mixed powder specimen and then drying the specimen at 100°C in a dryer. The measurement conditions were 20 scans, 4 cm−1 resolution, and 400~4000 cm−1 range. Also, the ESCALAB250 XPS system and the Theta Probe XPS system using monochrome Al Kα (hν = 1486.6 eV) radiation were used to analyze the state change of the V and Fe ions of the glass; the conditions of X-ray energy, sample positioning of the X-ray, and sampling time for all 4 specimens according to the crystallinity condition were maintained for the measurements. The analysis range was 400μm and the data were calibrated based on C1s (284.6 eV). The error of the peak position with suitable signal to noise ratio was approximately ± 0.2 eV.
A Keithley electrometer was used to measure the resistivity of each specimen at the temperature of 45°C; this was done so that the dc electrical conductivity for each specimen could be measured. Also, in order to investigate the catalytic property of the glass specimen for the oxidation reaction of fatty acids (linoleic acid and stearic acid), 10 mg of fatty acid and 10 mg of glass powder were mixed to obtain 2 specimens for the TGA analysis. The TGA analysis used the Q600 device manufactured by TA Instruments to carry out measurements from room temperature to 600°C with a heating rate of 10°C/min under a 5 vol% N2 gas atmosphere. The fatty acids used in the experiment were technical 60-74% (GC) grade linoleic acid and reagent 95% grade stearic acid manufactured by Sigma-Aldrich Co.
3. Results and Discussion
3.1. Density change
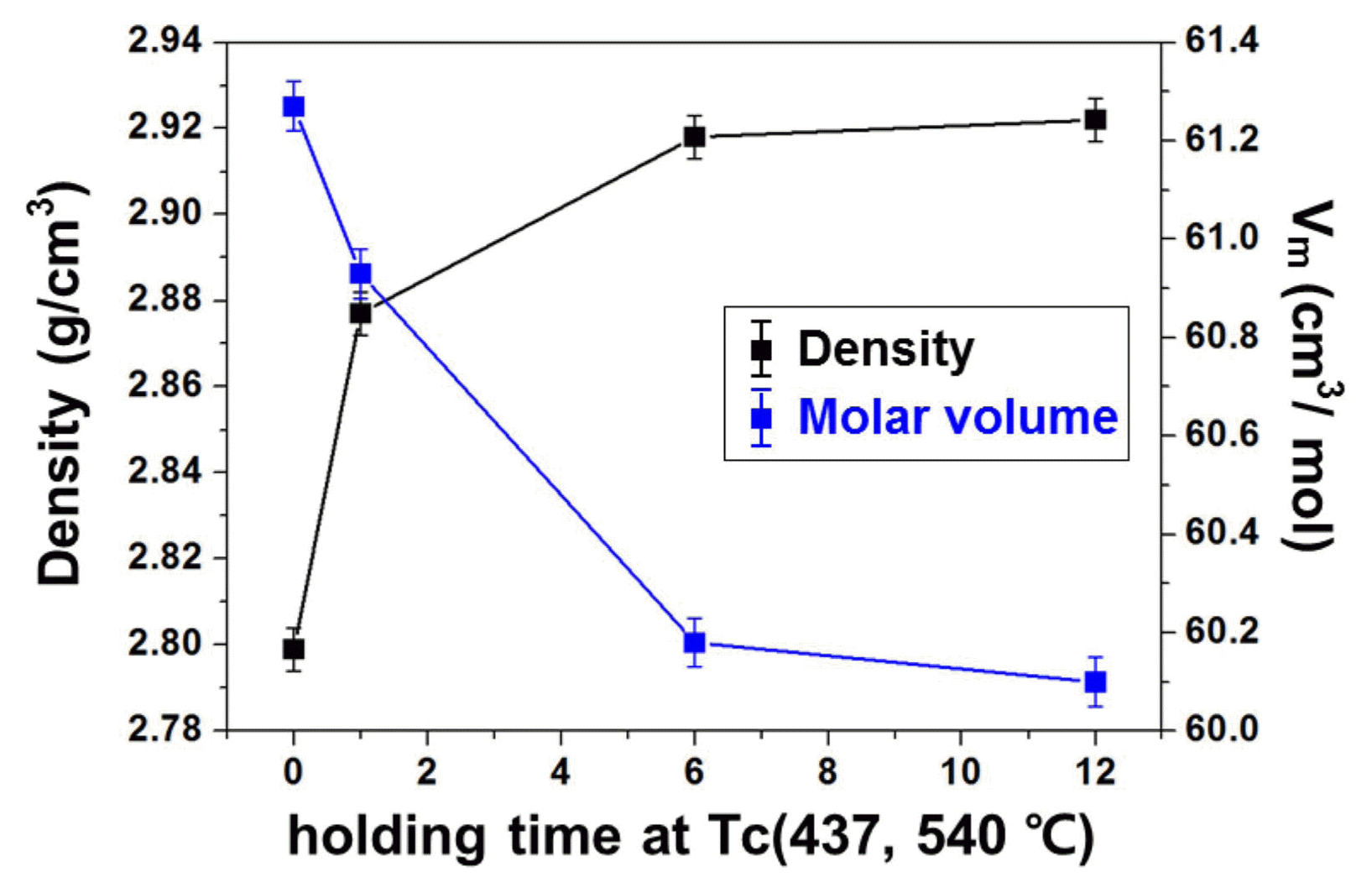
Figure 2 shows the density measurement of the glass specimen after annealing and heat treatment, as well as the molar volume obtained using the Archimedes method. In Fig. 2, it can be observed that the density and molar volume of the glass specimen changed, indirectly showing that the glass structure changed due to crystallization. Moreover, as the heat treatment time increased, the density increased, while the molar volume decreased. These changes were caused by thermal relaxation, which resulted in a decrease in the glass structure deformation. The increase in density showed that the glass structure strengthened as the heat treatment time increased; glass structure became highly cross-linked. This structural change was observed through the XRD and FT-IR measurements discussed below.
3.2. Structural change
Figure 3 shows the XRD analysis results of the crystalline phase formation for all the specimens with varying heat treatment times. Fig. 3(a) shows the XRD pattern of one specimen; no crystallinity peak was observed. The peak at 25° was due to the amorphous halo characteristic. Fig. 3(b) shows the XRD pattern of the glass that was heat treated for 1 h at the crystallization temperature Tc (437°C & 540°C), defined through the DSC measurement (Fig. 1). Unlike the annealed specimen, this specimen showed a few crystallinity peaks of low peak strength. The V2O5 (JCPDS 01-0359) phase was observed as the major crystallinity phase and the VO2 (JCPDS 09-0142) and B2O3 (JCPDS 06-0297) crystallinity peaks were also identified. Figs. 3(c) and (d) show the XRD patterns of the specimens heat treated for 6 and 12 h, respectively. The Fe0.12V2O5 (JCPDS 049-0805) crystallinity phase, which was not found for the specimen with 1 h crystallization, was observed; through this, the formation of new bonding reaction Fe-O-V was identified due to the bonding reaction between the different metal ions.11) Especially, the new crystalline phase includes numerous oxygen defects because it is a compound that has non-stoichiometric bonding between the metal and oxygen ions; structural defects of transition metal oxides, similar to these oxygen defects, were predicted to significantly affect the catalytic property.
Figure 4 shows the infrared spectrum of the glass specimens, including those that only underwent annealing and those with various heat treatment times. For the only-annealed specimen, it was observed that amorphous BO3 and VO5 were major structural groups existing on the glass, while VO4 and BO4 groups increased within the glass structure as the heat treatment time increased at the Tp temperature.12)
The band located in the 1200-1600 cm−1 range corresponds to the B-O bond of the BO3 trigonal group; the band located in the 900-1200 cm−1 range corresponds to the BO4 tetrahedra group.13) As the heat treatment time increased, the band of the BO3 trigonal group gradually increased and became flat and the B-O bond corresponding to the BO4 tetrahedra group increased and formed a valley.14) In addition to the XPS results, which showed that the V5+ to V4+ ion content increased as the heat treatment time increased, the decrease in the V=O double bond within the glass structure and the change from BO3 to BO4 showed that the glass structure change signifies a strengthening of the glass structure through a decrease of nonbridging oxygen within the glass structure. The band located in the 750-900 cm−1 range results from the B-O-V-O-B or BO4 unit, and represents the interaction that forms a network between the VO4 and BO4 units; this shows the relationship of the structural strengthening to the heat treatment time increase.15) The band observed for the 430-550 cm−1 range results from the interaction of the Fe3+ ion of Fe2O3 and the V=O bond, both existing in the glass structure. This band was not observed for (a) with only annealing carried out but the valley was observed for the 470 cm−1 band of (b)~(d) as the heat treatment time increased. This shows that the Fe ion in the vanadium chain represents the interstitial or substitutional inserted structure; according to Baran et al., this corresponds to strong interaction between the Fe-O and V-O vibrations due to the coordination numbers of V and Fe forming a strongly cross-linked structure.16,17) Also, the Fe2O3 is of an interstitial or substitutional form in the V2O5 matrix, and this result is in agreement with previous works in the literature, which showed that substitution in the matrix site was possible.18)
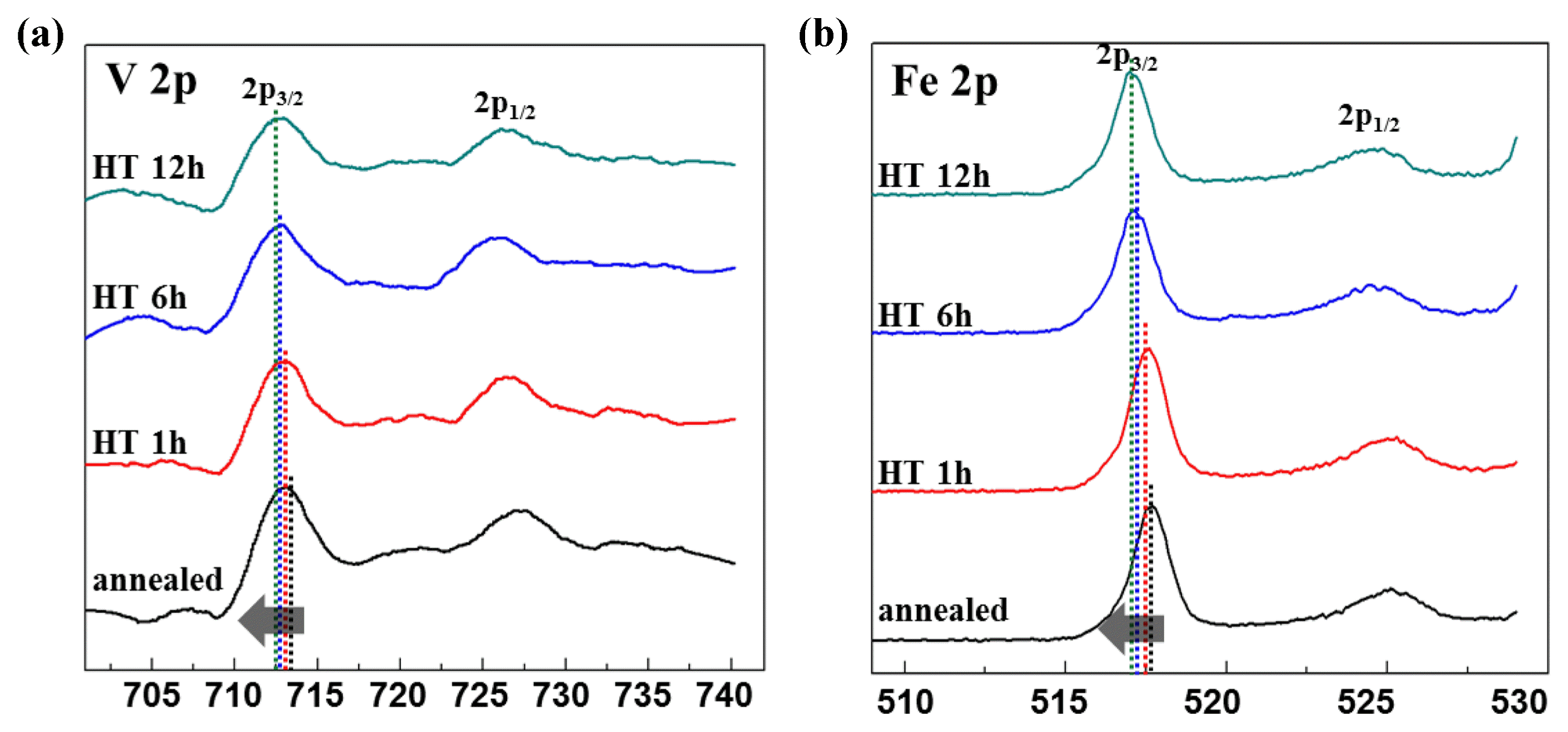
Figure 5 shows the results of XPS analysis, which was used to measure the doublet spectra of the V 2p and Fe 2p spin orbits in order to quantitatively evaluate the V and Fe ion oxidation number of the 70V2O5-10Fe2O3-13P2O5-7B2O3 glass. As shown in the figure, asymmetry was observed for both the V 2p and Fe 2p peaks, and this revealed that the V and Fe ions existed in two or more oxidation states.19)
According to a previous study on vanadium phosphate glass, the core level spectrum of V5+ showed a peak at the binding energy (BE) of 517.3 eV, while the peak level of V4+ was 516 eV.20) Thus, in accordance with the V 2p3/2 core level spectrum, the V5+ and V4+ ions were fitted according to the average binding energies of 517.3 eV and 516.1 eV, respectively, and the concentrations of V5+ and V4+ were quantitatively analyzed.21,22) Since Fe can exist in the forms of Fe2+ or Fe3+, the Fe 2p3/2 peak of the glass specimen can be decomposed into two Gaussian-Lorentzian peaks, as shown in Fig. 6; after fitting, fitting was carried out with Fe2+ and Fe3+ at ~ 711.7 and ~ 713.8 eV, respectively.23) The relative ratios V4+/Vtotal, V5+/Vtotal, Fe2+/Fetotal, and Fe3+/Fetotal, obtained through fitting, are shown in Table 1; it was observed that the V5+ and Fe2+ content decreased, while the V4+ and Fe3 content increased as the heat treatment time increased for the 70V2O5-10Fe2O3-13P2O5-7B2O3 glass crystallization.
3.3. Electrical conductivity and catalytic property change
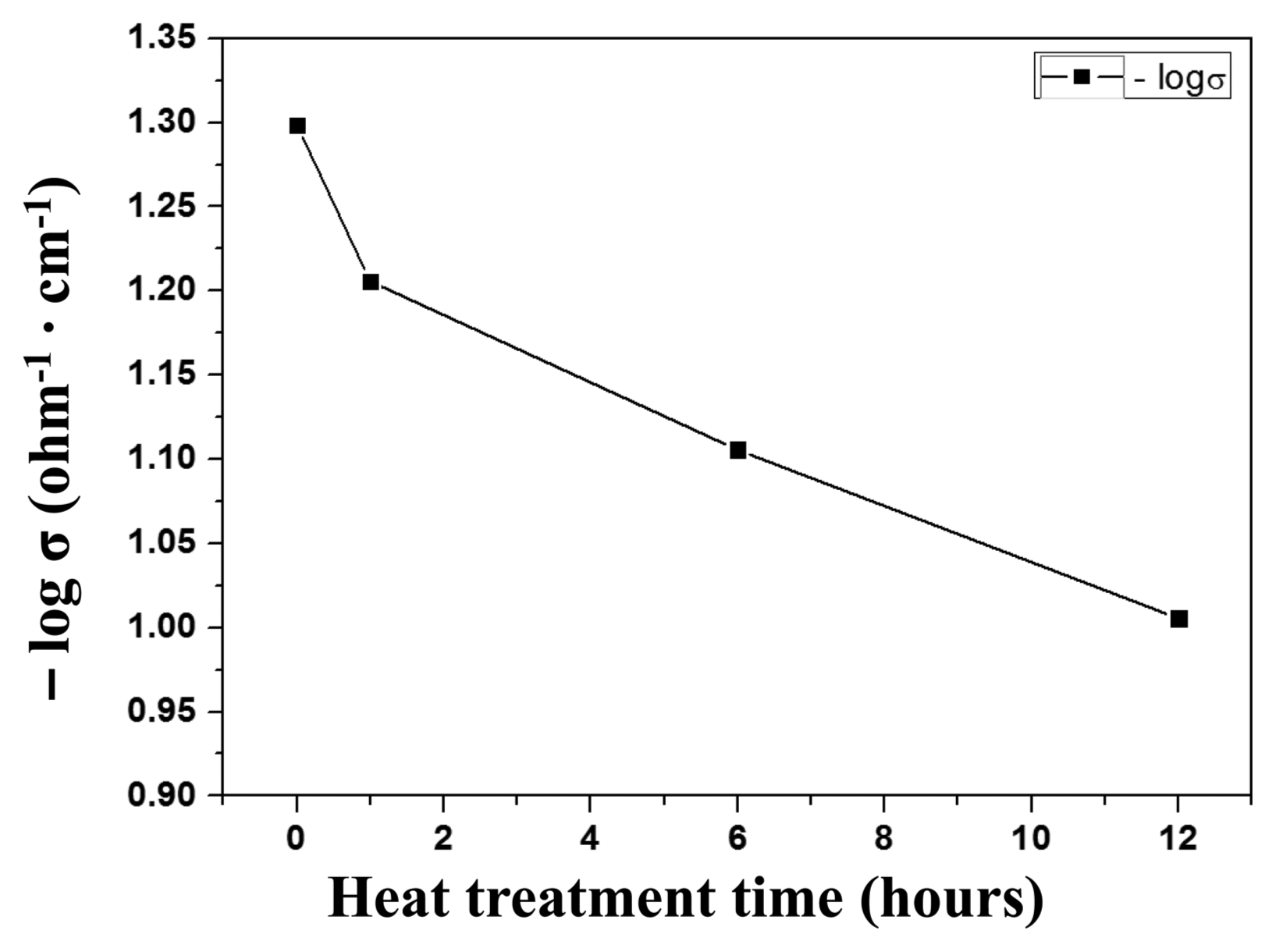
Figure 7 shows the resistivity measurement at 45°C for specimens with varying heat treatment times. The heat treatment time was varied among 1, 6, and 12 h. As the heat treatment time increased, the resistivity decreased and the electrical conductivity increased. This increase in electrical conductivity resulted from the densification of the structure. As the heat treatment time at the crystallization temperature increased, the crystal growth accelerated and the glass state turned to crystalline, causing structure densification. 18,19) The electrical conductivity within the glass can be explained using the small polaron hopping theory between the transition metal ions. The possible reaction processes in the system used in this study were Fe2+ → Fe3+, V4+ → Fe3+, and V4+ → V5+, where polaron hopping occurs from a low oxidation number ion to a metal ion with a high oxidation number, resulting in electrical conduction; the bonding of such metal ions creates conduction paths. These conduction paths affect the enhancement of electrical conductivity; this is in agreement with the vanadate glass electrical conductivity and other property studies by Gzowski et al., which utilized the electron transfer and bond formation between different metal ions such as V4+ and Fe3+ ions.24,25)
Moreover, the increase in the heat treatment time for crystallization leads to an increase in conduction paths and an increase in V4+ and Fe2, as shown in the XPS measurements (Fig. 6). Also, the increase in the Fe-O-Fe and Fe-O-V bonding found through the FTIR measurement supports this result.
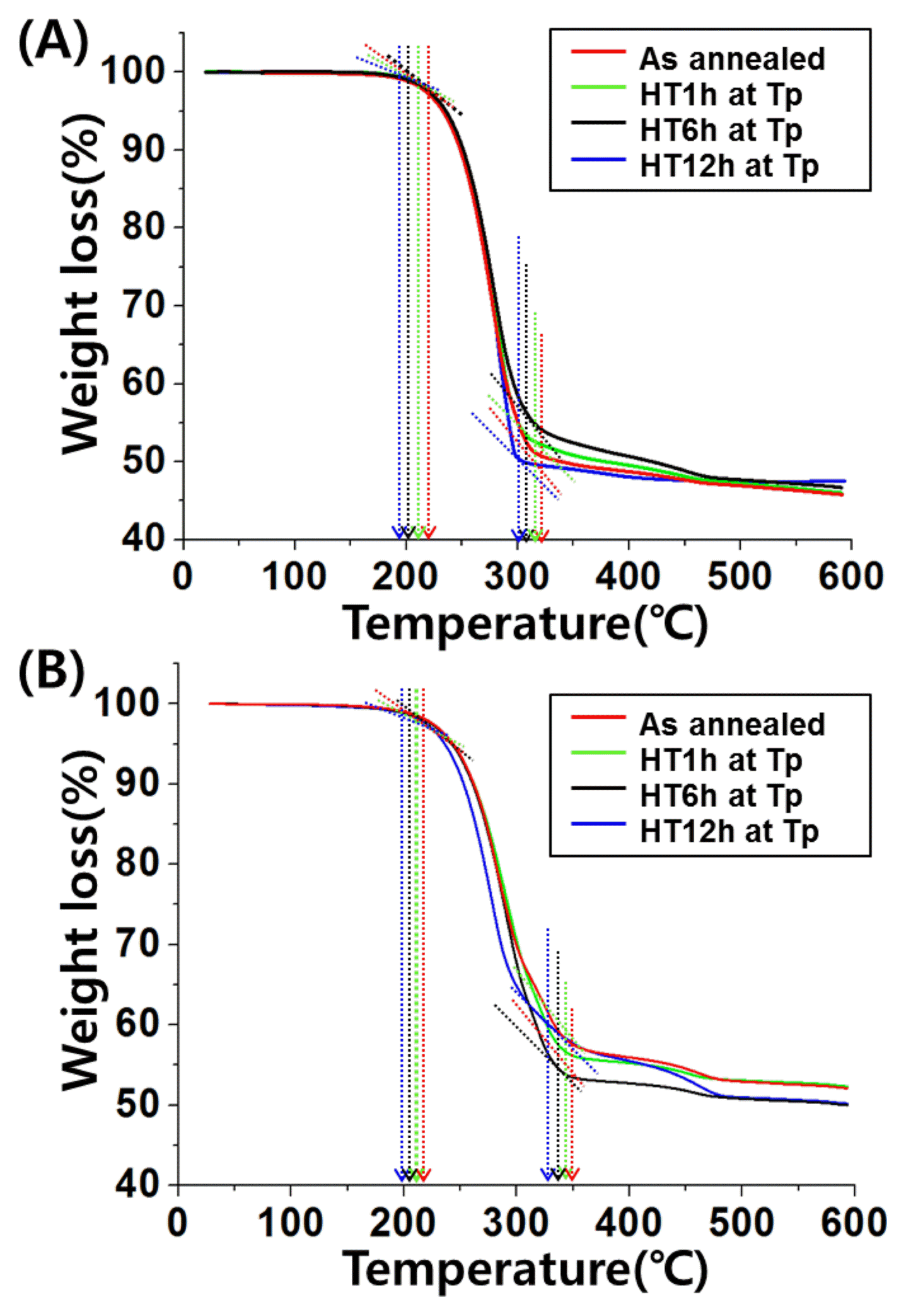
Figure 8 shows the TGA analysis results for the two sample types that mixed 10 mg of linoleic acid and stearic acid with 10 mg of glass powder obtained after crystallizing 70V2O5-10Fe2O3-13P2O5-7B2O3 glass under varying heat treatment conditions. Linoleic acid is a representative unsaturated fatty acid with two double bonds (CH3(CH2)4CH =CHCH2CH=CH(CH2)7CO2H); stearic acid is a representative saturated fatty acid with a carbon number of 18(CH3-(CH2)16COOH). Each fatty acid partially converts to CO2 gas through oxidation reaction, and mass loss occurs. The system glass used in this study underwent 1 h of heat treatment at Tp (437°C & 540°C), which was then followed by the formation of the crystalline phase. As a result, the temperature at which mass loss ends (according to the conversion of the linoleic acid and stearic acid to CO2 gas from the oxidation reaction) or the temperature at which the oxidation reaction of each fatty acid ends, decreases (Stearic acid 345.69~328.38°C, Linoleic acid 400.21~394.85°C). Afterwards, the temperature of mass loss termination was measured for the increased heat treatment times of 6 and 12 h; results revealed that the termination temperature gradually decreased. As shown in Table 2, the mass loss termination temperatures of the 12 h heat treated samples for stearic acid and linoleic acid were 301.33°C and 352.51°C, respectively. These results show that through the formation of a non-stoichiometric binder phase Fe0.12V2O5 could be observed through XRD measurement; it was determined that transition metal ions with various valence states within the glass bonded with oxygen to create scattered oxygen vacancies, which act as active sites for the oxidation reaction of fatty acid. So, when the heat treatment time is increased, the number of oxygen vacancies increase to increase the number of reaction sites for the oxidation reaction; consequently, the reaction termination temperature gradually impacts the decrease. Furthermore, it was predicted that the electron transfer will be active between the same type of ions, like V5+ to V4+, and between different metal ions, like the reduction reaction from Fe4+ to Fe3+, which were quantitatively measured through XPS analysis; this transfer process promotes the oxidation reaction of fatty acids.
4. Conclusions
The objective of this study was to investigate and verify the relationships concerning property changes, including the catalytic property and electrical conductivity, from the perspective of the glass structure by crystallizing system glass 70V2O5-10Fe2O3-13P2O5-7B2O3, which includes two transition metal oxides. The characteristics of vanadate glass, as shown in the studies by Bogomolova3) & Kawamoto, 8) which revealed that the metal ion ratio value (C = V4+/[V4+ + V5+]) and changes in the glass structure affect the electrical conductivity and catalytic property, were directly and indirectly observed through XRD, FTIR, XPS, and density measurement of the glass structure changes caused by crystallization.
Through the molar volume and density measurement results, it was found that the glass density increased with extended heat treatment times due to intensified crystallization within the glass, which caused structural densification. XRD analysis revealed that VO2, V2O5, B2O3, and Fe0.12V2O5 phases were formed by the crystallization. As the heat treatment time for crystallization increased, the VO2 phase increased, the V2O5 phase decreased, the V4+ content increased, and the V5+ content decreased. Also, when heat treatment was carried out for 6 h or more, the new binder phase Fe0.12V2O5 was formed, revealing the formation of bonding between Fe and V; this was also observed through bonding structure analysis through FTIR measurement, in which it was found that Fe-O-V bonding increased with increased heat treatment time.
Furthermore, the non-stoichiometric Fe0.12V2O5 phase formed through heat treatment contained numerous defects thus, many oxygen vacancies are formed within the glass and then act as active sites that promote an oxidation reaction for the decomposition of fatty acids in the crystallized glass. This was observed through TGA measurement results of the fatty acid mass loss termination temperature.