1. Introduction
β-SiAlON (Si6-zAlzOzN8-z), a solid solution of silicon nitride (Si3N4) showing excellent high-temperature strength, thermal shock resistance, oxidation resistance, and chemical stability, is applied to high-performance bearings, turbine blades, and non-ferrous molten metal refractories, because the volumetric ratio of the intergranular amorphous phase remaining after sintering is smaller than that of silicon nitride.1-2) In β-SiAlON, with an increase of temperature (1400 to 1600°C) following the conversion of the raw materials into a eutectic liquid, the low-temperature phase, α-Si3N4, melts into the liquid. β-SiAlON is then precipitated as a stable phase to accomplish densification through a transient liquid sintering mechanism, where crystallization occurs, theoretically, without a residual liquid.3,4) Generally, however, a certain amount of a sintering aid is required for high-density sintering of β-SiAlON, and the presence of a residual amorphous phase is unavoidable in the sintered body. Nevertheless, the high-temperature properties of the β-SiAlON-based sintered body are superior to those of the silicon nitride, because the amount of the added and/or remnant sintering aid is smaller in the β-SiAlON-based sintered body.
β-SiAlON, whose eutectic liquid contributing to densification is consumed by crystallization, is known as a poor sin-terable ceramic in comparison with silicon nitride. Methods of obtaining a high-density sintered body include pressureless sintering with the addition of an excessive amount of a sintering aid5) and press sintering with the addition of a minimum amount of a sintering aid. Examples of the latter are hot press (HP) sintering, hot isostatic press (HIP) sintering, and spark plasma sintering (SPS).6)
β-SiAlON is commonly prepared by reaction sintering where the synthesis from the starting materials, such as Si3N4, AlN, and Al2O3, occurs simultaneously with the densification of the SiAlON phase.7) However, since the non-oxide ceramics, e.g., Si3N4 or AlN, are expensive, the unit price of the sintered parts manufactured by using these materials is very high.8) In the present study, to enhance the price competitiveness of β-SiAlON parts, a β-SiAlON sintered body was prepared by a normal sintering process, not reaction sintering, by using a less expensive β-SiAlON powder manufactured by Self-propagating High-temperature Synthesis (SHS). SHS, which is a method of synthesizing a powder by using the heat of reaction released from the process of generating products through the reactions between raw materials, may enable low-price powder synthesis because the synthesis time is short and the required equipment is simple as the reactions are propagated and continued within the powder batch spontaneously without external energy.9) In the present study, pressureless sintering was performed in a nitrogen atmosphere by adding 0 to 7 wt% of Y2O3 as a sintering aid, and the properties of the resulting sintered body were compared with those of the sintered body produced by the costly reaction sintering.
2. Experimental Procedure
The starting raw materials were commercially available SHS β-SiAlON powder (Combustion Synthesis Co., Ltd, Hokkaido, Japan), represented by the chemical composition formula of Si6-zAlzOzN8-z (z = 0.5), and Y2O3 (grade C, H.C. Starck, Goslar, Germany) used as a sintering aid. The β-SiAlON powder was calcinated in air at 1000°C for 2 h, but weight loss due to residual organic substances was not detected. Wet ball-milling was performed after adding 0 wt%, 1 wt%, 3 wt%, 5 wt%, and 7 wt% of Y2O3 to the β-SiAlON powder (Table 1). The ball-milling was performed for 24 h at 200 rpm for 24 h by using an anhydrous alcohol solvent, a zirconia container, and Si3N4 balls having a diameter of 5 mm. The mixed powder was dried by using a rotary evaporator and then sieved by using a 230-mesh sieve. Uniaxial press molding was performed by weighing 1.2 g of the mixed powder and using a 15 mm round mold. Cold isostatic pressing at 200 MPa was then performed to prepare a green body having a diameter of about 14 mm and a height of about 4.5 mm. A graphite crucible was filled with an atmospheric powder (Si3N4 : BN = 1 : 1) to which the molded body was buried. The crucible was then charged to a graphite-heated furnace for sintering at 1800°C for 2 h (T18t2h) or for 4 h (T18t4h) in an 0.1 MPa nitrogen atmosphere. The former number in each of the experiment denotations refers to the sintering temperature, while the latter number refers to the duration. After the sintering, the density of individual specimens was measured by Archimedes method. In addition, a phase analysis of the mixed powder and the sintered specimens was performed through X-ray diffraction (D/MAX 2500VL, Rigaku, Japan). The specimens were ground to be 1.2 mm thick, and both surfaces were mirror-polished by using 1 μm diamond slurry to measure the biaxial flexural strength according to the ISO 6872 method. In addition, according to the method of ISO 6507-1, the Vickers hardness was measured by applying 1 kg of load (FM-700A, Future Tech, Japan) and the fracture toughness was measured by applying 20 kg of load (HV-112, Akashi, Japan). The average hardness and fracture toughness measurement values, obtained by performing the tests 7 and 9 times, respectively, were used for the analysis, and the fracture toughness was calculated by using the Niihara equation.10) In addition, the wear resistance of the T18t4h sintered body was measured by the ball-on-disk method (ASTM G133-05) at room temperature. The counterpart ball made of bearing-class silicon nitride was used in the test where the specimens were worn under a load of 5 N, in a wearing radius of 3 mm at a rate of 1000 rpm for 30 minutes, and then the amount of wear was analyzed by using an optical profiler (Tencor, P-1, NJ, USA). The polished sintered body was plasma-etched, and was then subjected to a micro-structure analysis using a scanning electron microscope (SEM, JSM-5800, Jeol, Japan) and X-ray spectroscopy using an energy dispersive spectrometer (EDS).
3. Results and Discussion
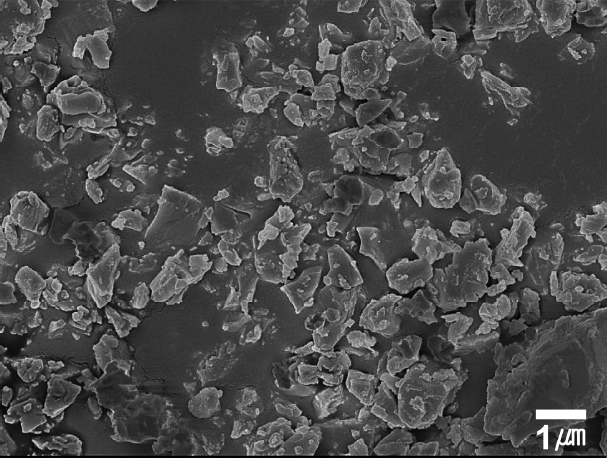
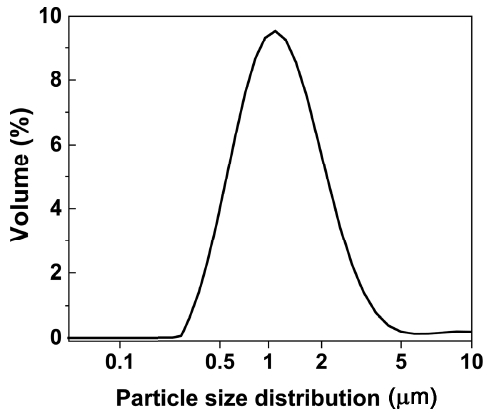
Figures 1 and 2 show SEM images and the particle size analysis results of the β-SiAlON powder synthesized by SHS. The analysis of the β-SiAlON powder showed that the powder had an angular shape due to the pulverization following the synthesis and a particle diameter of about 1 μm (D50 = 1.0 μm). Fig. 3 shows the relative density (%TD) of the sintered body depending on the amount of the added sintering aid and the sintering conditions (T18t2h and T18t4h). The average relative density of the specimens prepared by adding a sintering aid was over 98%, which was higher than that of the specimens without an sintering aid (below 92%). The densification and the phase transition rate are known to be dependent on the type and amount of the added sintering aid.11) In the present study, a sintered body having an excellent sintering density was obtained when Y2O3 was added at 3 wt%. On the other hand, the difference in the relative density depending on the sintering duration was negligible, indicating that the densification was completed by the sintering for 2 h.
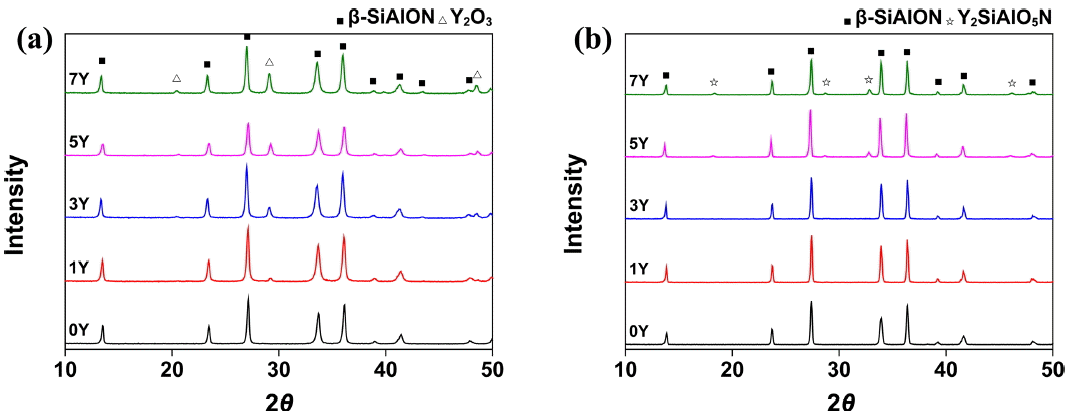
Figure 4(a) shows the XRD patterns of the β-SiAlON powder and the mixed powder. A powder synthesized by SHS is generally known to contain unreacted reactants even after the reaction process.12) The powder used in the present study was identified as a single β-SiAlON phase, which is presumed to be evidence of a purification process that followed the SHS synthesis. Fig. 4(b) shows the XRD pattern of the specimen prepared under the T18t4h condition. A secondary phase of Y2SiAlO5N was detected in the specimen prepared by adding the sintering aid at 5 wt% or higher. The secondary phase was particularly distinctive in the 7Y specimen, indicating that the secondary phase may be generated when a sintering aid is added excessively, as described above. Table 2 shows the EDS analysis results of the sintered body prepared under the T18t4h condition. The z-values of the specimens, representing the atomic ratio of Si : Al, were 0.65, 0.67, 0.63, 0.61, and 0.6, respectively, which were slightly higher than the substitution ratio of the purchased powder (0.5). The increase in the z-value was likely due to the increase of the stable phase of β-SiAlON as it was precipitated during the grain growth by the ‘dissolution-reprecipitation’ mechanism after a eutectic liquid was produced from the starting material, SiAlON, and the sintering aid, Y2O3. At the same time, the z-value decreased as the amount of the added sintering aid increased, possibly because the amount of substituted Al decreased due to the generation of a secondary phase including Al.
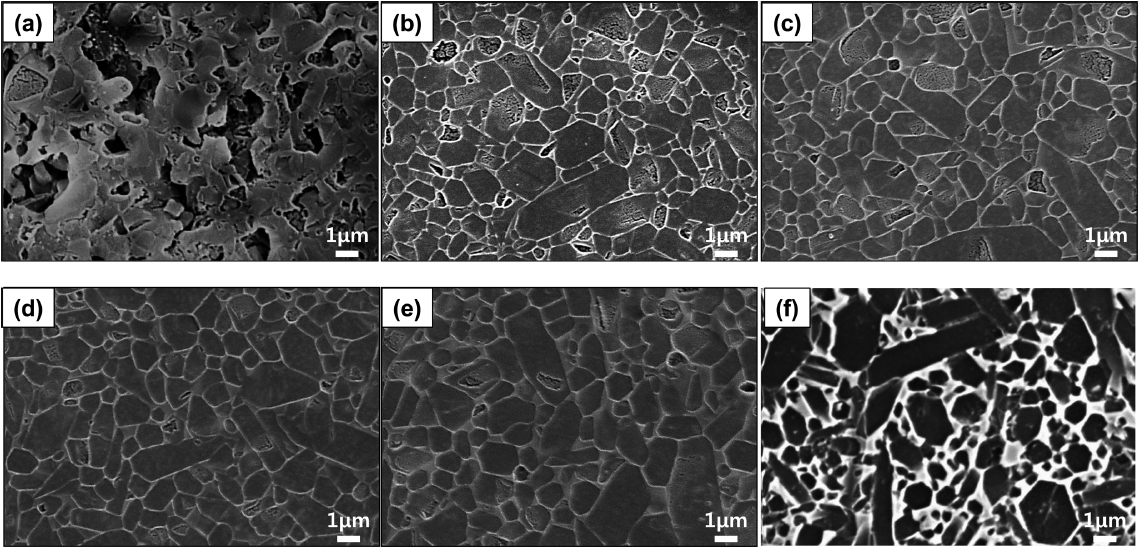
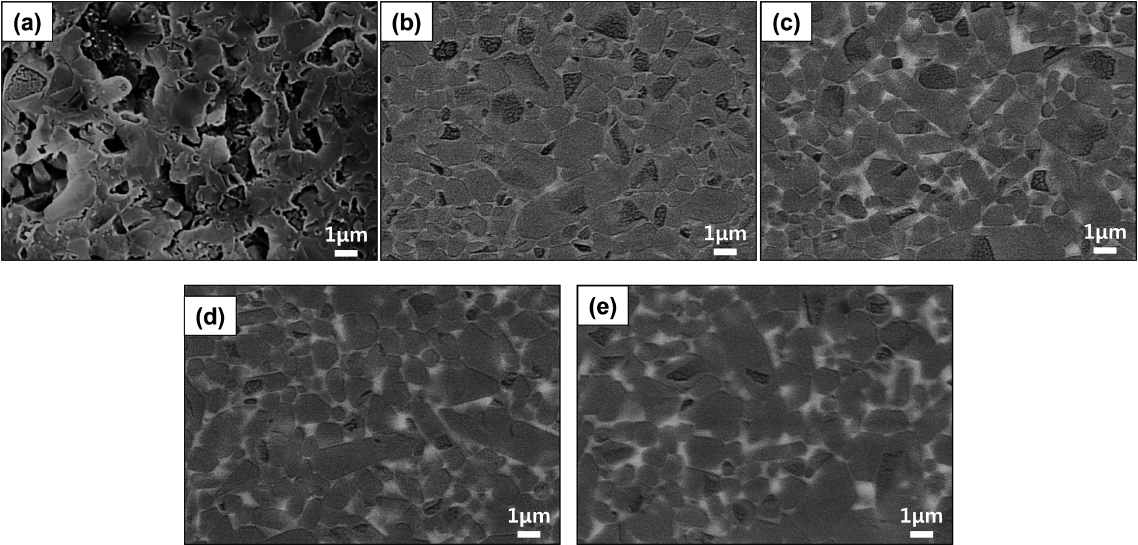
Figures 5(a) to 5(e) show the microstructure of the T18t4h sintered body observed in a secondary electron (SE) mode depending on the amount of the sintering aid. As the amount of the sintering aid increased, the size of the coarse particles remained unchanged but the size of the smaller particles decreased in a particle growth pattern. The cross-section of the coarse particles was close to hexagonal, and their size was about 3 μm. One notable finding in the present study is that the particle aspect ratio of the sintered body of the present study prepared by using the β-SiAlON powder as the starting material was smaller than that of the sintered body prepared by reaction sintering. Fig. 5(f) shows the microstructure of the specimen prepared by reaction sintering in our previous study.13) In the reaction sintering where α-Si3N4 is used as the starting material, a microstructure with a high aspect ratio may be obtained through the phase transition and particle growth by the dissolution-reprecipitation mechanism. However, in the present study where β-SiAlON was used as the starting material, abnormal growth of the particles leading to a large aspect ratio did not occur. Figs. 6(a) to 6(e) show the microstructure of the sintered body observed in a back-scattered electron (BSE) mode, indicating that the volume fraction of the intergranular amorphous phase increased as the amount of the added Y2O3 increased. Since the generation of an excessive intergranular amorphous phase decreases high-temperature mechanical properties and strength,14) a smaller amount of an amorphous phase is preferable at a level that enables densification.
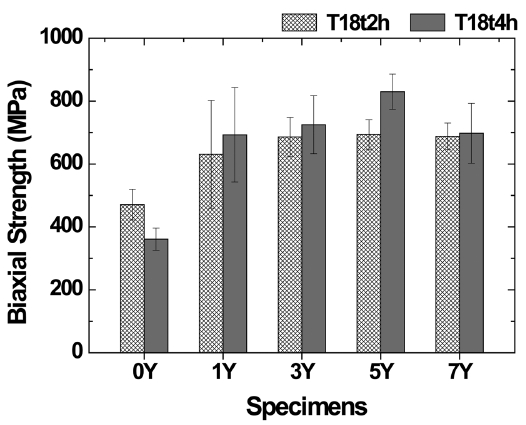
Figure 7 shows the biaxial flexural strength of the sintered body, indicating that the strength was dependent on the amount of the sintering aid. The optimal flexural strength (830 MPa) was found in the 5Y specimen. However, the flexural strength drastically decreased in the 7Y specimen containing 7 wt% of the sintering aid. This suggests that the generation of a large amount of a secondary phase having weaker mechanical properties may have decreased the strength.
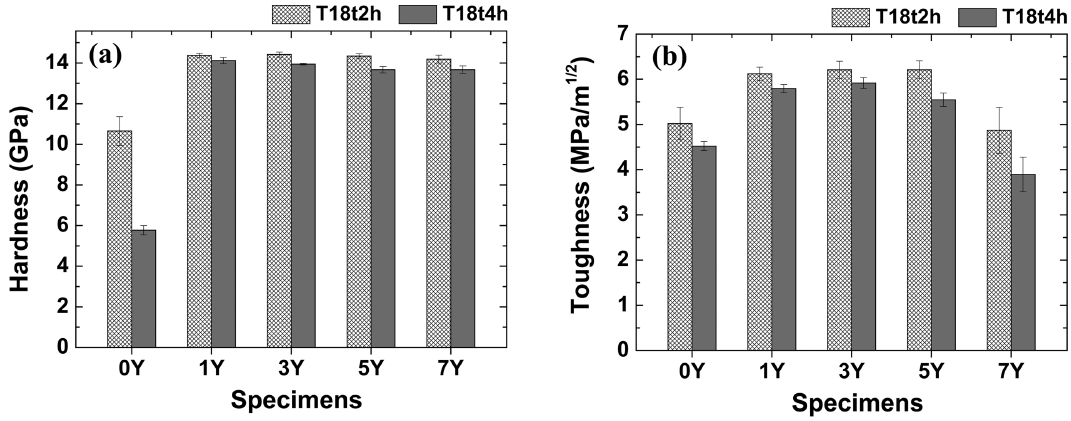
Figure 8 shows the hardness and fracture toughness of the T18t2h and T18t4h specimens depending on the sintering time and the amount of the sintering aid. The fracture toughness was calculated by using the following Niihara equation:
where KIC denotes the fracture toughness, E the elastic modulus, HV the hardness, P the indentation load, and C the crack length. The hardness and the toughness of the T18t2h specimen were higher than those of the T18t4h specimen, which indicates that excellent mechanical properties may be obtained by sintering for just 2 h, as described above. The hardness of all the specimens, except the 0Y specimen, was over 14 GPa, and the hardness slightly decreased as the amount of the sintering aid increased. The fracture toughness of the T18t2h specimens was equal to or higher than 5 MPa·m1/2, but it drastically decreased as the amount of the sintering aid increased. This may be because the volume fraction of the intergranular amorphous phase, having a lower hardness, increased and a secondary precipitant was generated when an excessive amount of the sintering aid was added. On the other hand, the strength and the toughness of the sintered body prepared in the present study were lower than those of the abovementioned sintered body prepared by the conventional reaction sintering (strength: 1050 MPa; toughness: 6.08 MPa·m1/2).13) The decrease of the strength in comparison with the sintered body prepared by the reaction sintering may be because of the increase of the average particle size, and the decrease of the toughness may be due to the decrease of the particle aspect ratio.15)
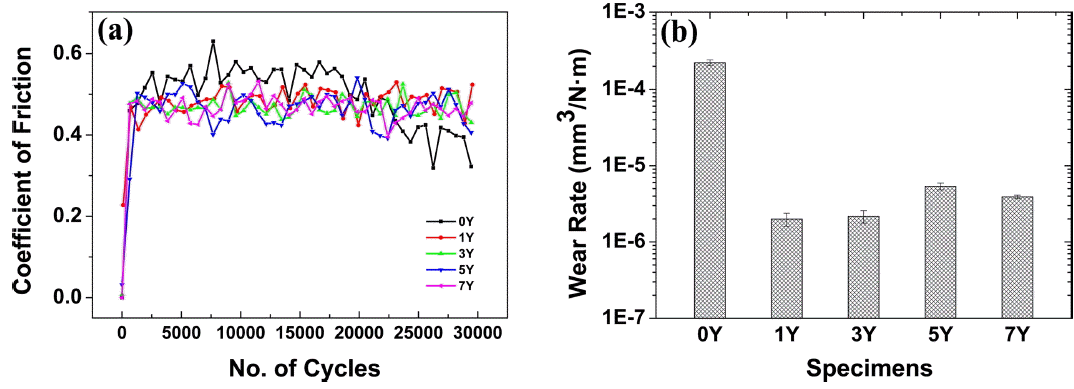
Figure 9(a) shows the coefficient of fraction (COF) of the sintered body. The COF remained constant in a range from 0.4 to 0.6 regardless of the amount of the added Y2O3. However, the 0Y specimen having a low density was rapidly worn as the number of cycles increased. Fig. 9(b) shows the wear rate representing the amount of wear divided by the load and the wear distance. An average wear resistance of 3 × 10−6 mm3/N·m was accomplished in all the specimens, except the 0Y specimen. As the amount of the sintering aid increased, the wear resistance decreased but within the same order of magnitude. However, since the addition of an excessive amount of the sintering aid may cause the formation of a glass-like phase at the triple point as well as detachment due to the increased volume fraction and wear focused on the detached region, an optimal amount of Y2O3 from 1 wt% to 3 wt% may be appropriately added to improve the wear resistance.
4. Conclusions
In the present study, a β-SiAlON sintered body was prepared for the first time in Korea by a normal sintering process, not reaction sintering, by using a β-SiAlON powder synthesized by SHS. The following findings were made through this study. First, a dense sintered body having a relative density of over 98% was obtained under pressureless sintering conditions at 1800°C for 2 h. Second, since the powder prepared by SHS already had the β-SiAlON phase, the aspect ratio of the grains obtained by the normal sintering was smaller than that of the grains prepared by the conventional reaction sintering where the synthesis starts with an α phase to obtain a β phase. Third, the addition of an excessive amount of Y2O3, a sintering aid, lowered the mechanical properties of the sintered body because of the generation of an intergranular amorphous phase and a secondary phase, and the appropriate content of the Y2O3 addition was found to be 3 wt% to 5 wt%. Fourth, although less expensive β-SiAlON powder was used, various mechanical properties of the sintered body, except the strength value, were similar to those of the expensive sintered body prepared by conventional reaction sintering. The results of the present study may be applied to realize low-cost SiAlON ceramics having excellent price competitiveness, and the sintered body prepared in the present study may be applied to various mechanical parts.