1. Introduction
MOFs1) are a novel type of functional materials, which are formed by metal ions or metal clusters and organic ligands. Owing to the remarkable properties of MOF materials, such as adjustable structure, low density, and high specific surface area, they will have important applications in the gas storage, catalysis, sensing, separation, and biomedical fields, among others, and are becoming one of the hot spots in the field of materials and chemical engineering.2-3)
At present, there are many types of MOF synthesis; however, for most of these methods, it is difficult to maintain the structure in an unchanged form in practical applications, such as those that take place in media such as acids, alkalis, and water for long times at high temperature. The structural stability of MOFs is becoming an important problem hindering the application of the application of industrialization to these materials.4-5) Regarding the structural stability of zirconium metal organic framework materials, the heat resistance of UIO-66 (with terephthalic acid as the ligand), synthesized by Cavka,6) was 773 K; this material also has good water and acid resistance, but its structure is destroyed under alkaline conditions. Further research has found that functionally modified form of UiO-66,7,8) such as UiO-66-NH2 and UiO-66-NO2, showed significantly decreased heat resistance at temperatures in the range of 623-673 K, and in hydrochloric acid (HCl,1pH) and sodium hydroxide (NaOH, 14pH) solutions; the main MOF structures of UiO-66-NH2, UiO-66-Br, and UiO-66-NO2 appeared partially to collapse, or to completely collapse. So far, most of the studies9-12) that have been reported are about the chemical stability of UIO-66 in media of water, HCl, and NaOH; however, only seldom has the chemical stability of UIO-66 in other acids and alkaline environments been studied.
Compared with terephthalic acid, the ligand of 9,10-dicarboxylic acid anthracene (H2DCA), which involves two more molecules benzene, was added by forming a larger conjugated _ bond to greatly improve the hydrogen adsorption enthalpy (Qst)13) and further enhance the new type of hydrogen storage properties of MOFs. The big conjugate system of the anthracene ring makes the benzene ring on both sides exist in the high density of the electron cloud, which has high reactivity; this is better for making further chemical modification to UIO-66(H2DCA).
Based on the above, we synthesized a new type of zirconium metal organic framework material, UIO-66 (H2DCA), with 9, 10-dicarboxylic acid anthracene as ligand and Zr4+ as the central ion; we studied its stability under different conditions in order to better use this material for hydrogen storage materials.
2. Experimental Procedure
2.1 Synthesis and activation of UIO-66 (H2DCA)
9,10-dicarboxylic acid anthracene was synthesized according to the process listed in the references.14) The ZrCl4 (0.985 mmol) and acetic acid (1.5 mL) were dissolved in DMF (15 mL), which was recorded as solution A. 9,10-dicarboxylic acid anthracene (0.822 mmol) and triethylamine (0.5 mL) were dissolved in DMF (20 mL); this was recorded as solution B. Then, solution B was slowly added to solution A; this was followed by adding of triethylamine (2 mL) to the reaction solution, which was maintained at 318 K for 15 h. After the reaction, the yellow solid was filtered and repeatedly washed with DMF; it was then immersed in a 323 K water bath in acetone for 3 days. Finally, the yellow solid was filtered, dried under vacuum at 393 K, and kept in a sealed bag; this resulting material was named UIO-66 (H2DCA)-1. At the same time, the samples that were subjected to solvent thermal reaction were named UIO-66 (H2DCA)-2; these samples had the same raw material ratio as that of UIO-66 (H2DCA)-1. Moreover, the chemical stability of UIO-66 (H2DCA)-1 was tested under different conditions.
2.2 Characterization of chemical stability
Samples of zirconium metal organic framework material UIO-66(H2DCA)-1 were immersed in hydrochloric acid, sulfuric acid, nitric acid, phosphoric acid solution (5 mL,1 mol/L) and sodium hydroxide solution (5 mL,1 mol/L), ammonia solution, triethylamine, and ethylenediamine (5 mL) at 298 K for 24 h. The same amounts of samples were immersed in two high pressure reaction kettles with hydrochloric acid (5 mL, 1 mol/L) and water at 373 K for 5d; then, the product was filtered and dried.
The thermal stability of the materials was measured using a synchronous TG-DSC thermal analyzer (STA-409PC Netzsch, heated to 1173 K at a rate of 5K/min under air gas). To check functional group changes on the samples under different environmental conditions via attenuated total reflectance (ATR) technique, FTIR spectra were obtained using an FTIR instrument (Nicolet 6700, 4000 ~ 500 cm−1 scans range, 2 cm−1 resolution and 16 scans).
SEM analysis (JSM-7500F JEOL) was used to capture and determine the morphologies of the crystalline UIO-66(H2DCA) samples. X-ray powder diffraction patterns were obtained with an X-ray diffractometer (MiniFlex 600, Cu Ka radiation, λ= 1.5406 Å, 0.02° step size); patterns were used to investigate changes of the crystal structure. N2 adsorption (JW-BK132F) was used to determine the pore size and surface area of the UIO-66 (H2DCA) samples. The samples were evacuated at 393 K under high vacuum for 2 h prior to the adsorption measurements.
3. Results and Discussion
3.1 Structural analysis of UIO-66 (H2DCA)
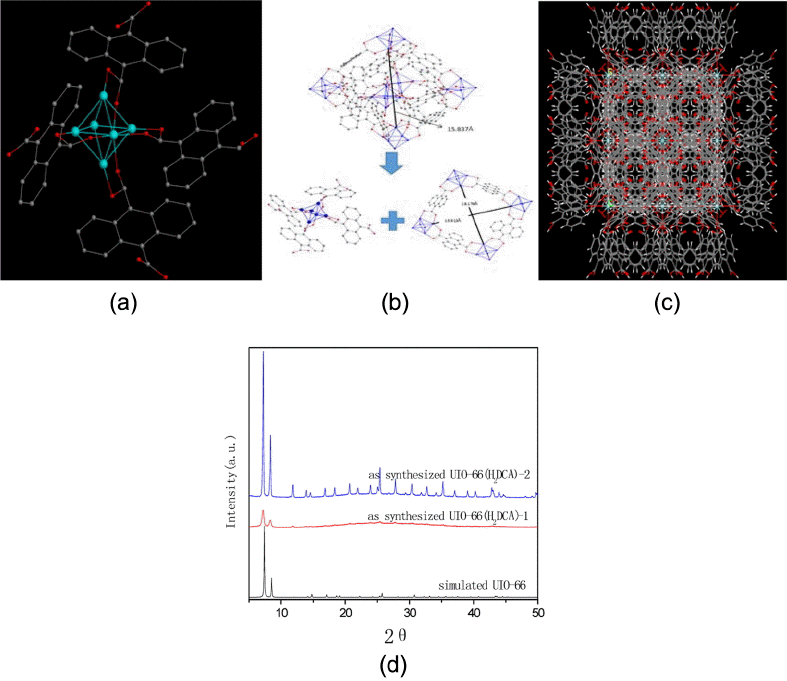
The way to investigate the ideal crystal structure of UIO-66 (H2DCA) is as shown in Fig. 1.
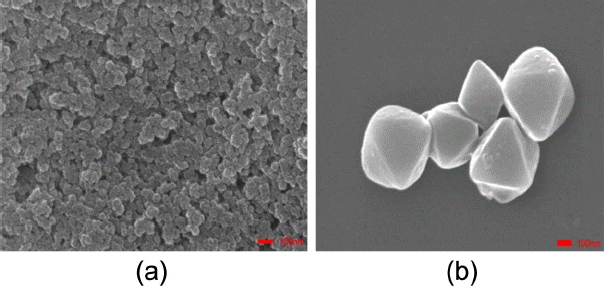
Each SBU of UIO-66 (H2DCA) is coordinated by twelve 9,10-dicarboxylic acid anthracenes and four ligands, as shown in Fig. 1(a), The distances between the zirconium atoms of the SBUs and the parameter of octahedrally shaped pores are 15.837 Å, 15.913 Å, and 16.179 Å, as can be seen in Fig. 1(b); the SBU and the ligands forming the 3D structure of UIO-66 (H2DCA) are shown in Fig. 1(c). From Fig. 1(d), it can be seen that the XRD patterns of the UIO-66 (H2DCA) are the same as those of the UIO-66, as has been reported before.6) This suggests that UIO-66 (H2DCA) and UIO-66 have similar crystal structures with good crystallization. The crystalline form of UIO-66(H2DCA)-2, synthesized with solvent thermal treatment, is better than the crystalline form of UIO-66(H2DCA)-1. That is say, the crystal grains of UIO-66(H2DCA)-2 are larger and more complete. The crystal sizes of UIO-66(H2DCA)-1 and UIO- 66(H2DCA)-2 were captured and determined by SEM, as shown in Fig. 2. The crystallite size of UIO-66(H2DCA)-2 was similar to an octahedral shape at about 500 nm, but the particle size of UIO-66 (H2DCA)-1 was found to have dropped; this was confirmed by XRD.
3.2 Thermal stability analysis of UIO-66 (H2DCA)-1
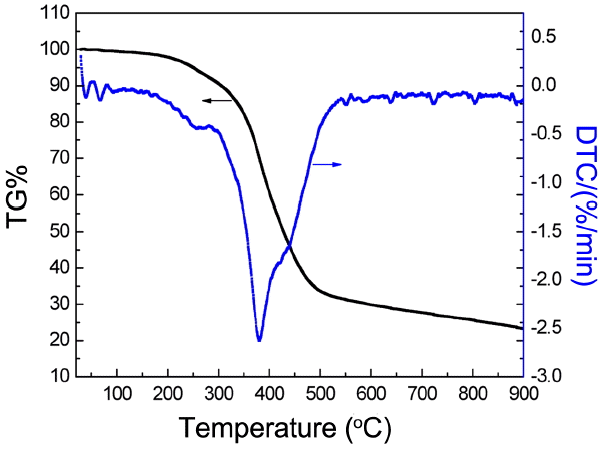
Thermal stability analysis of UIO-66 (H2DCA)-1 was conducted using a TG-DSC, with results as shown in Fig. 4. It is clear that four stages of weight loss occurred for UIO-66 (H2DCA)-1. According to the thermal decomposition process, it can be deduced that the first weight loss at around 323 K should be attributed to the volatilization of acetone; the second weight loss at around 353 K is due to the evaporation of DMF and surface adsorbed water; the third weight loss at about 523 K is due to the beginning of scission of about coordination key chain of Zr-O and the CO2 decomposition of the 9, 10 - anthracene dicarboxylic acid ligand; and the fourth stage of weight loss at 583 K-773 K is in virtue of the further decomposition of UIO-66 (H2DCA)-1 to form carbonaceous material and ZrO2. It is obvious that the thermal stability of UIO-66 (H2DCA)-1 can reach 583 K. The thermal stability data for UIO-66 (H2DCA)-1 and other MOF materials is listed in Table 1. From Table 2, we can see that UIO-66 (H2DCA)-1 has a better thermal stability.
3.3 Chemical stability of UIO-66 (H2DCA)-1
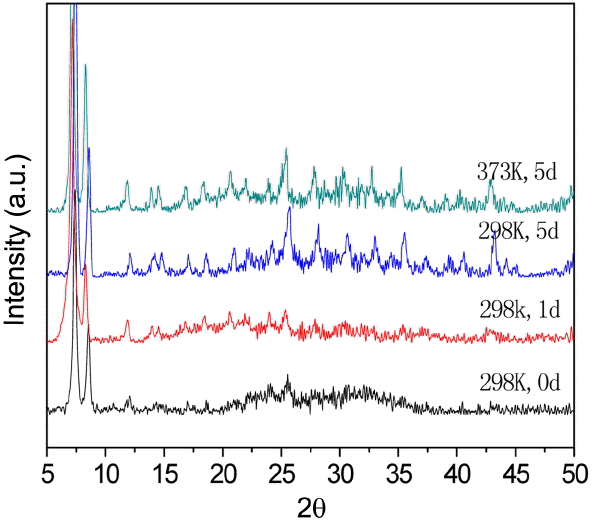
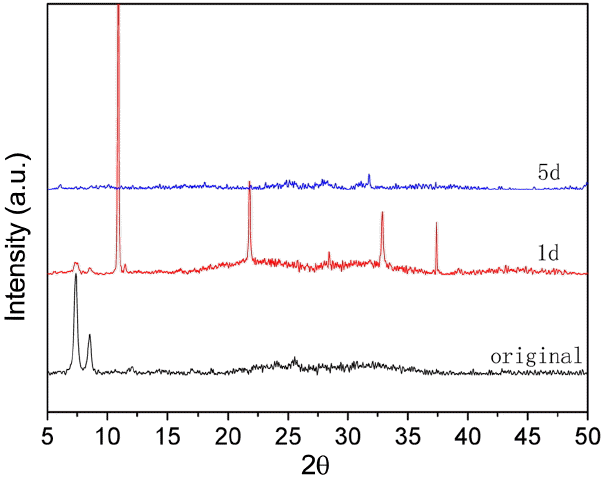
The stability of UIO-66 (H2DCA)-1 in water was checked by XRD, with results shown in Fig. 5. It can be seen that the UIO-66 (H2DCA)-1 crystal structure is unchanged when sample is put in boiling water; that is, the UIO-66 (H2DCA)- 1 sample has a high water resistance.
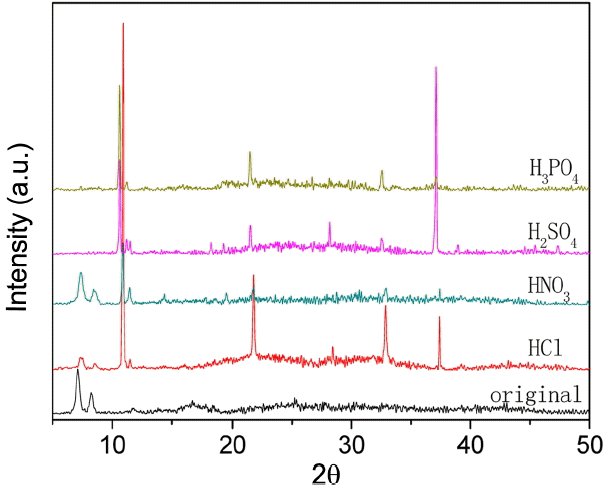
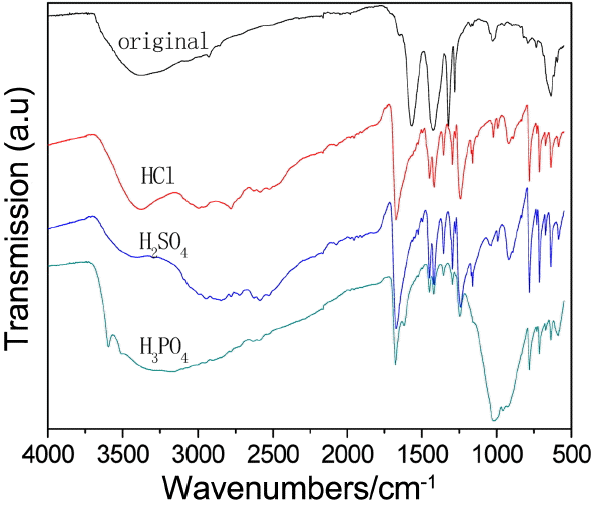
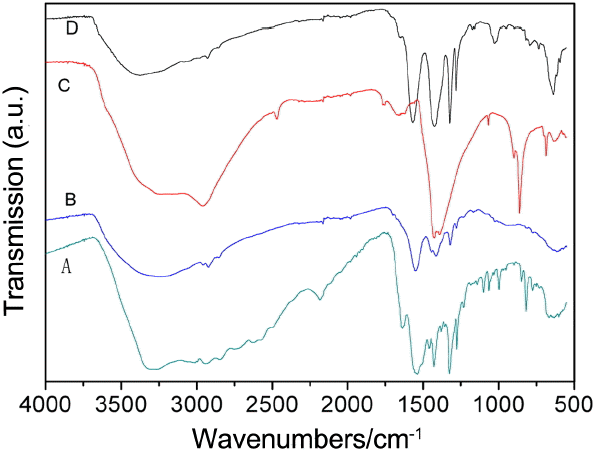
Figure 6 displays PXRD patterns of UIO-66 (H2DCA)-1 in different acid environments. The stability of UIO-66 (H2DCA)-1 in hydrochloric acid was investigated by XRD, with results as shown in Fig. 7. It can be seen that the structure of UIO-66 (H2DCA)-1 is unable to remain stable in hydrochloric acid and, with increasing time, its structure is gradually destroyed and finally completely decomposes. The structure of UIO-66 (H2DCA)-1 is known to be incapable of maintaining its stability in hydrochloric acid due to the distribution of Cl−1. Compared with SO4−2 and PO4−3 ions, the Cl−1 ion takes a very long time to destroy the structure of UIO-66 (H2DCA)-1 completely. Fig. 8 provides the FTIR spectra of the UIO-66 (H2DCA)-1 sample after acid treatment. It can be clearly seen that, after treatment with hydrochloric acid, sulfuric acid, and phosphoric acid, the structural damage was decomposed into the 9,10- two carboxylic acid of H2DCA and zirconium salt. It should be recognized that a broad IR band in the region of 2500-3000 cm−1 occurred owing to the carboxyl hydroxyl. The peak at 1676 cm−1 belongs to the stretching vibration of carbonyl. It is clear that the peak at 1017 cm−1 is due to PO43− vibration, as shown Fig. 6(d). As a result of the strong coordination ability of Cl−1, sulfate ion and phosphate ion were able to destroy the original Zr-O coordination bond; that is, the UIO-66 (H2DCA)-1 sample was decomposed into 9, 10- dicarboxylic acid anthracene and the corresponding zirconium salt.
As is known to all, UIO-66 (H2DCA)-1 is a zirconium oxygen coordination compound. It is clear that the structure of UIO-66 (H2DCA)-1 is stable in nitric acid, slightly damaged in hydrochloric acid, and completely destroyed in sulfuric acid and phosphoric acid; these conditions obtain because nitrate ion cannot provide a lone pair electrons to form a zirconium coordination bond, and UIO-66 (H2DCA)-1 is naturally stable in nitric acid. Because the electronegativity values of phosphorus and sulfur are less than that of oxygen, and because oxygen has a strong attraction for electrons, it is easy to contribute a lone pair of electrons to oxygen to form the coordination bond, that is, SO4−2 and PO4−3, with a zirconium ion form the zirconium phosphate coordination bond and the zirconium sulfate coordination bond; the original Zr4+ and carboxylic (-OOC) coordination bond is completely destroyed in sulfuric acid and phosphoric acid. The conditions obtain because the levels of electronegativity of chloride ions and oxygen ions are relatively close, and the coordination ability of the chloride ion is not strong enough. In the presence of Cl−1 with zirconium ion, it is more difficult to form zirconium chlorine coordination than it is to form SO4−2 or PO4−3 with the zirconium ion. Thus, UIO-66 (H2DCA)-1 exhibits different levels of stability in different acidic media.
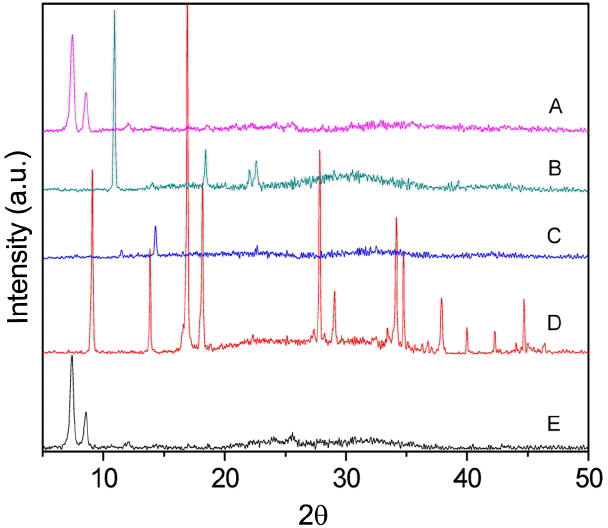
Figure 9 shows the XRD patterns of the UIO-66 (H2DCA)-1 sample in different alkali environments. It is obvious that the structure of the UIO-66 (H2DCA)-1 sample was completely destroyed by the sodium hydroxide solution, the ammonia solution, and ethylenediamine. The Zr-O coordination bond is easily disrupted because the volume of hydroxide ion is small and this ion has strong coordination ability. The compound ethylenediamine is an organic alkali and, owing to its strong coordination ability for its two amino groups and its small steric hindrance, also easily causes the structure of UIO-66 (H2DCA)-1 to become unstable. It is very interesting that triethylamine is also an organic alkali, but the structure of UIO-66 (H2DCA)-1 still maintains its stability. This is because triethylamine finds it very difficult to enter into the MOF structure due to its large steric effect; thus, UIO-66 (H2DCA)-1 can be stable in triethylamine.
The FTIR spectra of the samples of UIO-66 (H2DCA)-1 after alkali treatment are shown in Fig. 10. The peaks at 686 cm−1, 867 cm−1, 1396 cm−1, 1425 cm−1, and 2966 cm−1 are attributed to zirconium hydroxide, as shown in Fig. 8(b). Compared with the data shown in Fig. 8(a), the peaks at 636 cm−1 and 1025 cm−1 disappear in Fig. 8(c) due to the ammonia solution, which can ionize a small amount of OH−, which may do great damage to the Zr-O coordination bond. The peaks at 821 cm−1, 1383 cm−1, 1462 cm−1, and 2169 cm−1 emerge owing to the strong basicity and small steric hindrance of ethylenediamine, which make it is easy to attack the Zr-O coordination bond.
Therefore, this research shows that the structure of UIO- 66 (H2DCA)-1 is easily destroyed by an inorganic alkali environment; however, larger organic alkali molecules may be beneficial to the stability of UIO-66(H2DCA)-1.
4. Conclusions
The UIO-66 (H2DCA)-1 material can keep its structure stable at 583 K; it can be stable in boiling water, nitric acid, and triethylamine; however, it is unstable in hydrochloric acid, sulfuric acid, phosphoric acid, sodium hydroxide, ammonia solution, and ethylenediamine. The structural stability of UIO-66 (H2DCA)-1 in an inorganic acid depends on the type of anion and the coordination ability with the zirconium ion. The stronger the coordination ability of the zirconium ions is, the more likely the alkali is to cause structural damage to UIO-66 (H2DCA)-1. An inorganic base can lead to the destruction of the structure of UIO-66 (H2DCA)-1 because the volume of hydroxide ions is small and the inorganic base has a strong coordination ability, which makes it to destroy the Zr-O coordination bond. Ethylenediamine is an organic alkali; its two amino groups have strong coordination ability and small steric hindrance, which also make it very easy to strike the Zr-O coordination bond, which can lead to the destruction of the UIO-66 (H2DCA)-1 structure. However, the larger organic alkali molecule of triethylamine is beneficial to the stability of UIO-66(H2DCA).-1